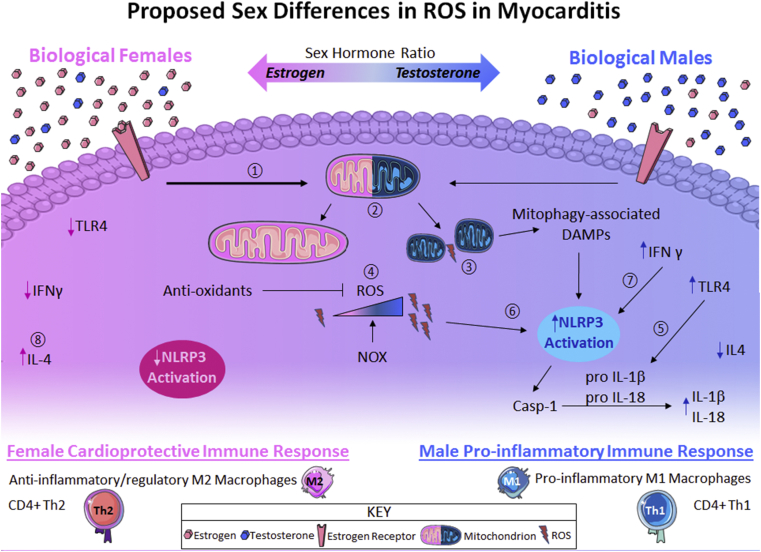
Fig. 3.
Proposed model of sex differences in ROS during myocarditis.① Increased ER activation due to higher estrogen in females leads to ② a profusion phenotype during myocarditis, whereas lower ER signaling in males during myocarditis leads to ③ increased mitochondrial fission. ④ Profusion mitochondrial phenotype in females decreases ROS while anti-oxidants neutralize excess ROS produced by NOX. In males, mitochondrial fission contributes to higher ROS while also releasing mitophagy-associated DAMPs (as mitochondrial fusion can be a precursor to mitophagy under cellular stress conditions such as infection) which can activate the inflammasome. ⑤ Increased TLR4 signaling in males compared to females leads to higher proIL-1β and proIL-18 which are cleaved by activated caspase-1 ⑥ to the active forms IL-1β and IL-18. ⑦ More inflammasome activation occurs in males due to increased intracellular ROS with higher IFNγ signaling compared to females. ⑧ Elevated IL-4 in females promotes a cardioprotective immune response comprised of anti-inflammatory Th2 CD4+ T cells and regulatory M2 macrophages while in males elevated IL-18 and IFNγ promote a proinflammatory immune phenotype resulting in M1 macrophages and Th1 CD4+ T cells.