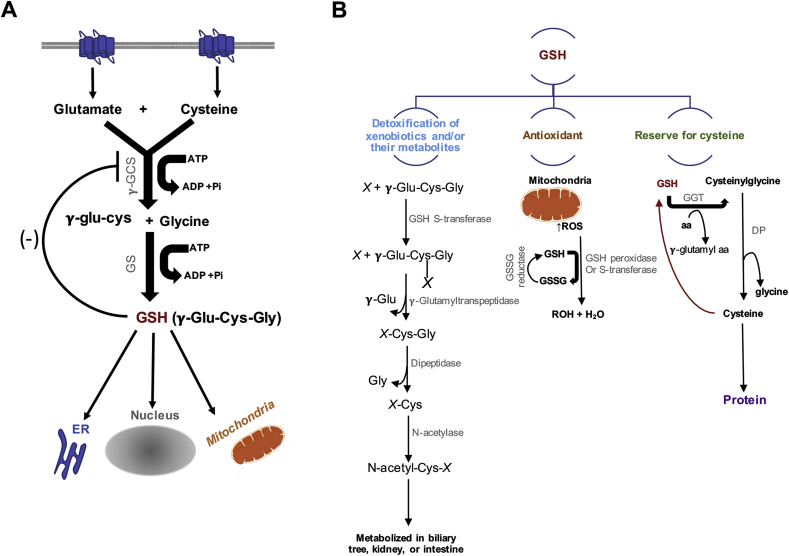
Fig. 1.
Glutathione (GSH) Synthesis and role in maintaining systemic redox balance. (A) GSH is synthesized from glutamine, cysteine, and glycine in the cytosol by an ATP-dependent two-step process: 1) This step conjugates cysteine with glutamate, generating γ-glutamyl cysteine (γ-glu-cys) by the action of γ-glutamyl cysteine synthase (γ-GCS, also known as glutamate–cysteine ligase (GCL)) and 2) glutathione synthetase then catalyzes the addition of glycine to γ-glutamylcysteine to form γ-glutamylcysteinylglycine (γ-Glu-Cys-Gly) or GSH. Increased levels of GSH activates a negative feedback inhibition. GHS is then distributed to different areas in the cell, such as the endoplasmic reticulum (ER), the nucleus, and the mitochondria. (B) GSH functions as a detoxification system, antioxidant, and it is the major reserve for cysteine. GSH conjugates with electrophile compounds spontaneously or via enzymatically in reactions catalyzed by GSH-S-transferase. These conjugates are cleaved by γ-glutamyltranspeptidase leaving a cysteinyl-glycine conjugate (X-Cys-Gly). The cysteinyl-glycine bond is then cleaved by dipeptidase. The remaining cysteinyl conjugate (X-Cys) is acetylated by N-acetylase leading to the formation of a mercapturic acid (N-acetyl-Cys-X). This conjugate is then metabolized in the biliary tree, intestine, or kidney. As an antioxidant, when reactive oxygen species (ROS) are produced (mainly by mitochondria), GSH peroxidase or GSH-S-transferase catalyzes the conjugation of GSH to ROS. Oxidized GSH (GSSG) and water are then formed. GSSG can be reduced back to GSH by GSSG reductase. GSH also serves as a source for cysteine. Gamma-Glutamyl Transferase (GGT) catalyze the transfer of the γ-glutamyl moiety of GSH to an amino acid (aa) forming γ-glutamyl amino acid and cysteinylglycine, which is broken down by dipeptidase (DP) to generate cysteine and glycine, which are then transported back into the cell and use for protein synthesis or GSH regeneration.