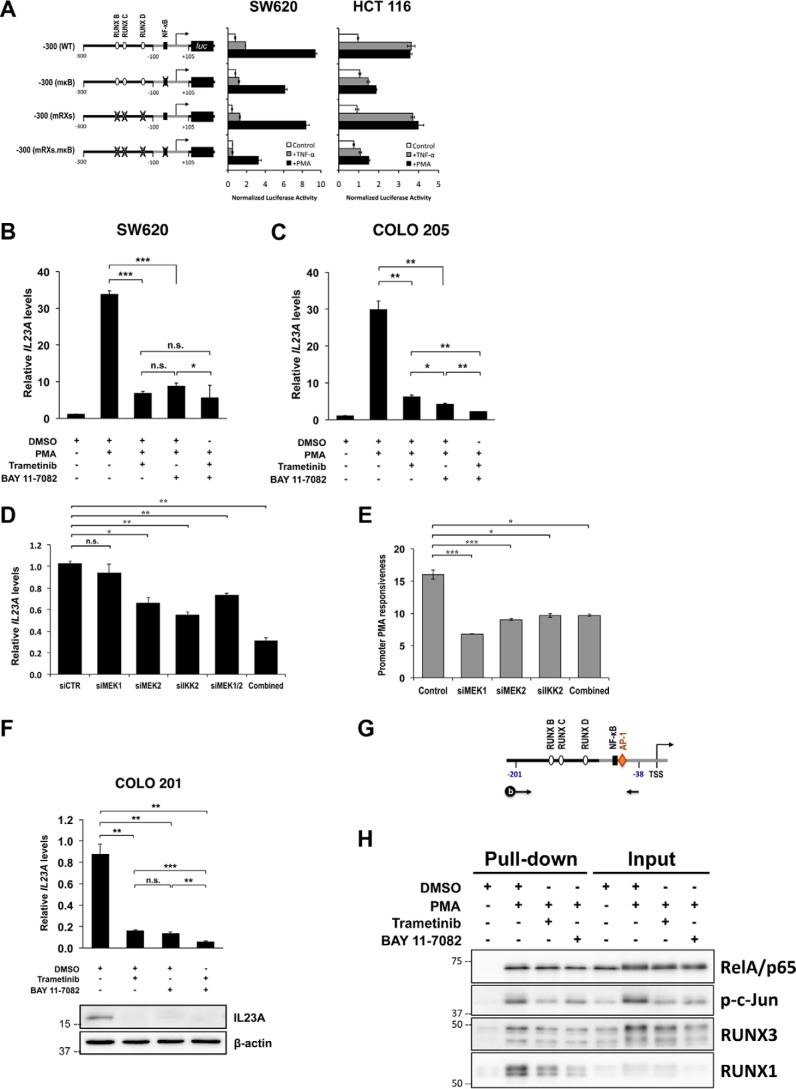
Figure 3.
Strong cooperation between the MAPK/MEK and NF-κB pathways in the transcriptional regulation of IL23A in intestinal epithelial cells. A, SW620 and HCT 116 cells were transiently transfected with WT reporter construct or variants with mutated RUNX and NF-κB sites (as indicated) and rested for 24 h. Subsequently, cells were treated with TNFα (50 ng/ml) or PMA (1 μm) for 8 h and harvested for luciferase assays. Normalized luciferase activities are expressed relative to those of untreated control samples (mean ± S.E.; n = 4). B and C, SW620 (B) and COLO 205 (C) were pretreated with either DMSO (Mock), trametinib (10 nm), BAY 11-7082 (10 μm), or in combination for 2 h before treatment with PMA (1 μm) or DMSO (Mock) for 24 h. Changes in IL23A mRNA levels were measured by qRT-PCR, normalized against corresponding GAPDH values, and presented relative to those of untreated controls (mean ± S.E.; n = 4). D and E, effects of RNAi of MEK1/MEK2 and the IKKα/β complex. D, SW620 cells were transfected with the indicated siRNAs. Cells were rested 36 h post transfection for effective RNAi knockdown and then treated with PMA for 10 h and harvested for qRT-PCR analyses. IL23A mRNA levels were normalized against 18S rRNA and expressed relative to the DMSO-treated control (siCTR) (mean ± S.E.; n = 3). E, WT −300 (WT) reporter construct was co-transfected with the indicated siRNAs into SW620 cells. Cells were rested 36 h post transfection for effective RNAi knockdown and then treated with PMA for 12 h and harvested for luciferase assay. The data presented are -fold increases in relative promoter activity following PMA treatment, relative to the DMSO-treated samples (mean ± S.E.; n = 3). F, COLO 201 were treated with DMSO (mock), trametinib (10 nm), BAY 11-7082 (10 μm), or in combination for 24 h and harvested for qRT-PCR measurements and Western blotting. Expression of IL23A mRNA was normalized against GAPDH values and charted relative to mock values (mean ± S.E.; n = 4). The p values are indicated as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001; n.s., not significant. G and H, promoter pulldown assay reveals a PMA-inducible transcription enhancer complex at the proximal IL23A promoter. G, schematics illustrating the coupling of a 5′ biotinylated amplicon spanning the −201 to −38 region of the IL23A proximal promoter to paramagnetic beads and used to enrich RelA/p65, p-c-Jun, RUNX3, and RUNX1 from nuclear extracts prepared from SW620 cells treated as indicated. H, PMA-induced nuclear p-c-Jun, RUNX3, and RUNX1 and their binding to the IL23A promoter fragment strongly enhanced RelA/p65 recruitment. Co-treatment with trametinib and BAY 11-7082 attenuated to varying degrees the induction of these transcription factors, thereby disrupting the formation of the transcription enhancer complex.