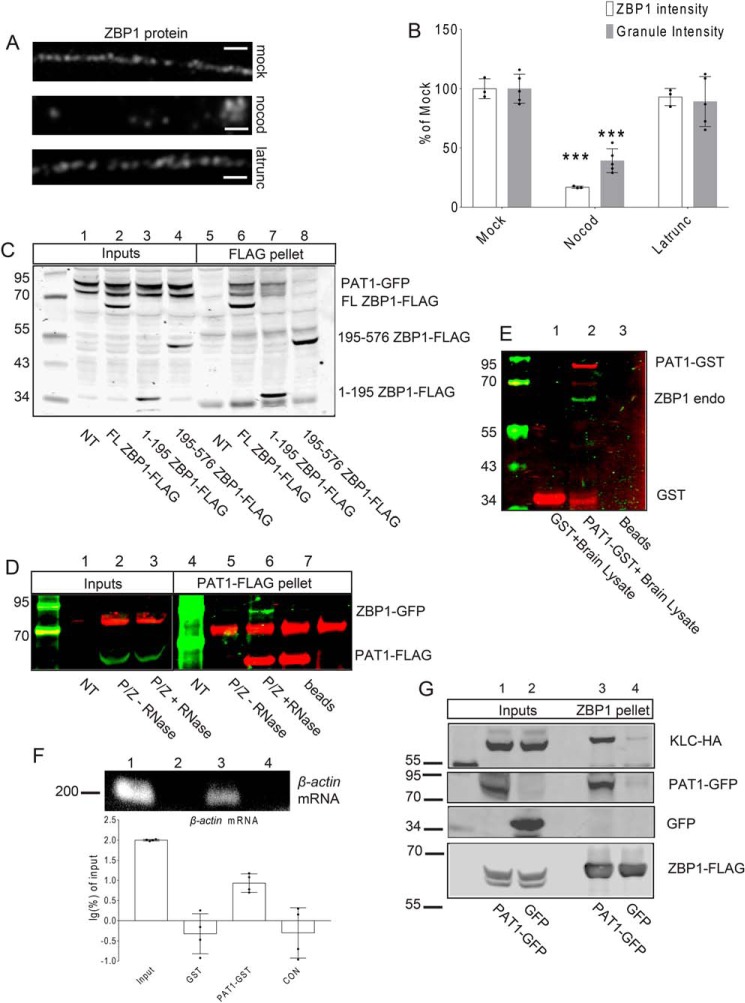
Figure 1.
A, mouse primary hippocampal neurons (7 DIV) were treated with the carrier (mock, upper panel), nocodazole (nocod, middle panel), or latrunculin (latrunc, lower panel) for 30 min; neurons were fixed and immunostained for ZBP1. Latrunculin has no effect on the distribution of dendritic ZBP1, whereas nocodazole treatment causes the reduced distribution of ZBP1 along dendrites. Scale bar, 5 μm. Three repeats were done independently with similar results for this experiment. B, histogram shows the quantification of dendritic ZBP1 intensities and granule intensities from A. Mock-, nocodazole (nocod)-, and latrunculin (latrunc)-treated dendrites are shown; nocodazole treatment decreases dendritic ZBP1 intensity, whereas latrunculin treatment has no effect on the distribution of ZBP1 along dendrites. ***, p < 0.001, n = 12 cells, two dendrites per cell, error bar, ±S.D. C, both the N-terminal and full-length (FL) ZBP1 interact with PAT1. Co-IPs were performed between PAT1–GFP and the full-length ZBP1–FLAG (FL ZBP1–FLAG, lanes 2 and 6) or the N terminus of ZBP1 (1–195 ZBP1–FLAG, lanes 3 and 7) or the C terminus of ZBP1 (195–576 ZBP1–FLAG, lanes 4 and 8) with anti-FLAG antibody-conjugated beads. NT denotes only PAT1–GFP-transfected cells (lanes 1 and 5). Both the full-length ZBP1 (lane 6) and the N terminus of ZBP1 (lane 7) are positive for interactions with PAT1–GFP. Six independent repeats were done for this experiment. D, interaction between PAT1 and ZBP1 requires RNA. ZBP1–GFP was co-transfected with PAT1–FLAG into HEK-293 cells (P/Z) and immunoprecipitated with anti-FLAG antibody-conjugated beads. ZBP1–GFP is precipitated by PAT1–FLAG in normal conditions (lane 5, −RNase), but not after RNase treatment (lane 6, + RNase). NT denotes nontransfected lysate; beads denote beads only; P/Z denotes transfected cell lysate. Six independent repeats were done for this experiment. E and F, recombinant PAT1–GST interacts with the endogenous ZBP1 protein and β-actin mRNA from mouse brain lysate. GSH–Sepharose-immobilized PAT1–GST interacts with the endogenous ZBP1 protein (E, lane 2) and β-actin mRNA (F, lane 3, 200 bp) from P0 mouse brain lysate. Histogram shows the log percent of β-actin mRNA associated with the recombinant PAT1–GST pellets, determined by RT-PCR. The amount of β-actin mRNA recovered from each group was normalized by the input and transformed into log percent of input. The result demonstrated that recombinant PAT1–GST interacts with endogenous ZBP1 and β-actin mRNA from the P0 brain lysate (F, lane1, lysate input; lane 2, GSH–Sepharose-immobilized GST only; lane 3, GSH–Sepharose-immobilized PAT1–GST; lane 4, CON denotes GSH–Sepharose only). Three independent experiments were performed. G, ZBP1 associates with KLC through PAT1. HEK-293 cells were co-transfected with KLC–HA, ZBP1–FLAG, and either GFP (lanes 2 and 4) or PAT1–GFP (lanes 1 and 3). Both lysates were immunoprecipitated with anti-FLAG antibody-conjugated beads. Blots indicate that the interaction between ZBP1–FLAG and KLC–HA requires the presence of PAT1–GFP (lane 3). Six independent repeats were done for this experiment.