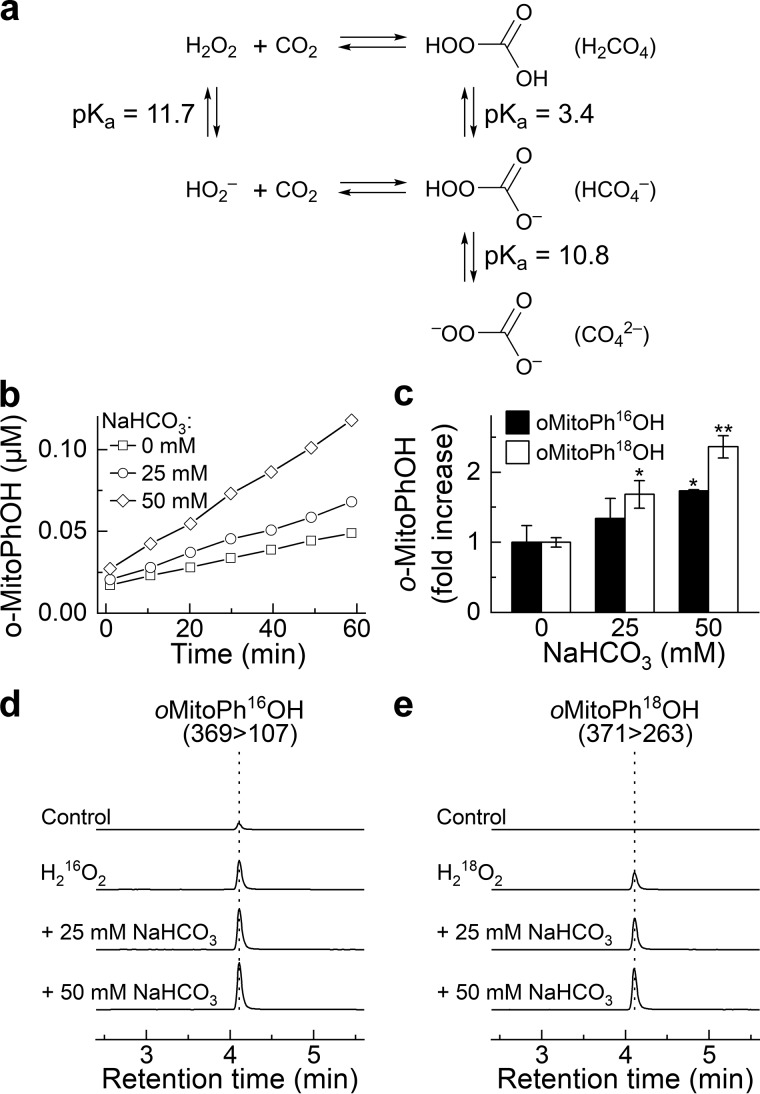
Figure 6.
NaHCO3-enhanced oxidation of oMitoPhB(OH)2 by H2O2 and incorporation of an oxygen atom from HCO4− into the phenolic product. a, chemical scheme of the formation of HCO4− and acid-base equilibria involved. b, dynamics of the formation of oMitoPhOH in the absence and presence of NaHCO3. c, relative increase in the yield of oMitoPhOH after 1-h incubation of the probe with H216O2 or H218O2 in the absence and presence of NaHCO3. d and e, LC-MS/MS traces of the phenolic products containing 16O (d) or 18O (e) atoms. LC-MS/MS analyses were performed after incubation (1 h) of oMitoPhB(OH)2 (1 μm) alone (control), with H216O2 (50 μm, d), or with H218O2 (50 μm, e). All solutions contained 0.1 m phosphate buffer and 0.1 mm dtpa, and the pH of the solutions was adjusted to 7.0.