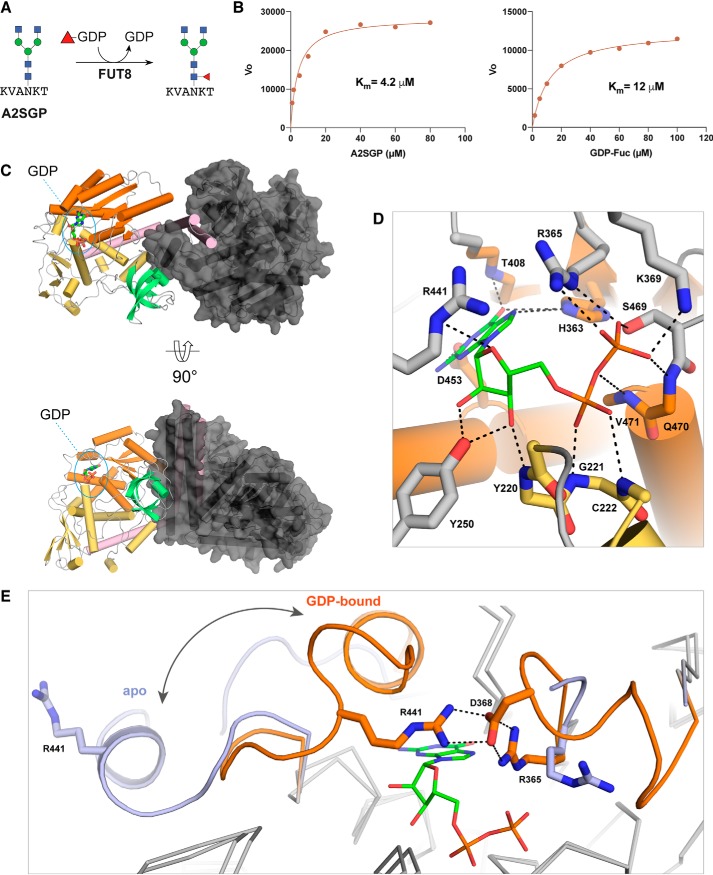
Figure 1.
Structures of mouse and human FUT8 with and without GDP bound. A, the reaction catalyzed by FUT8 in our GDP-Glo assay. B, HsFUT8 is active and has Km values that are in agreement with those reported previously (24). C, the domain structure of FUT8 (coiled coil, pink; Rossman, orange and yellow; SH3, teal) and the interactions between each the two molecules in the asymmetric unit. D, the hydrogen-bonding interactions between MmFUT8 and GDP (see also Fig. S4). E, an overlay of the GDP-binding sites of apo-HsFUT8 (blue) and GDP-bound MmFUT8 (orange), illustrating the conformational changes observed for loops A and B. A salt bridge between Asp368 and Arg365/Arg441 from loops A and B, respectively, forms upon encapsulation of GDP.