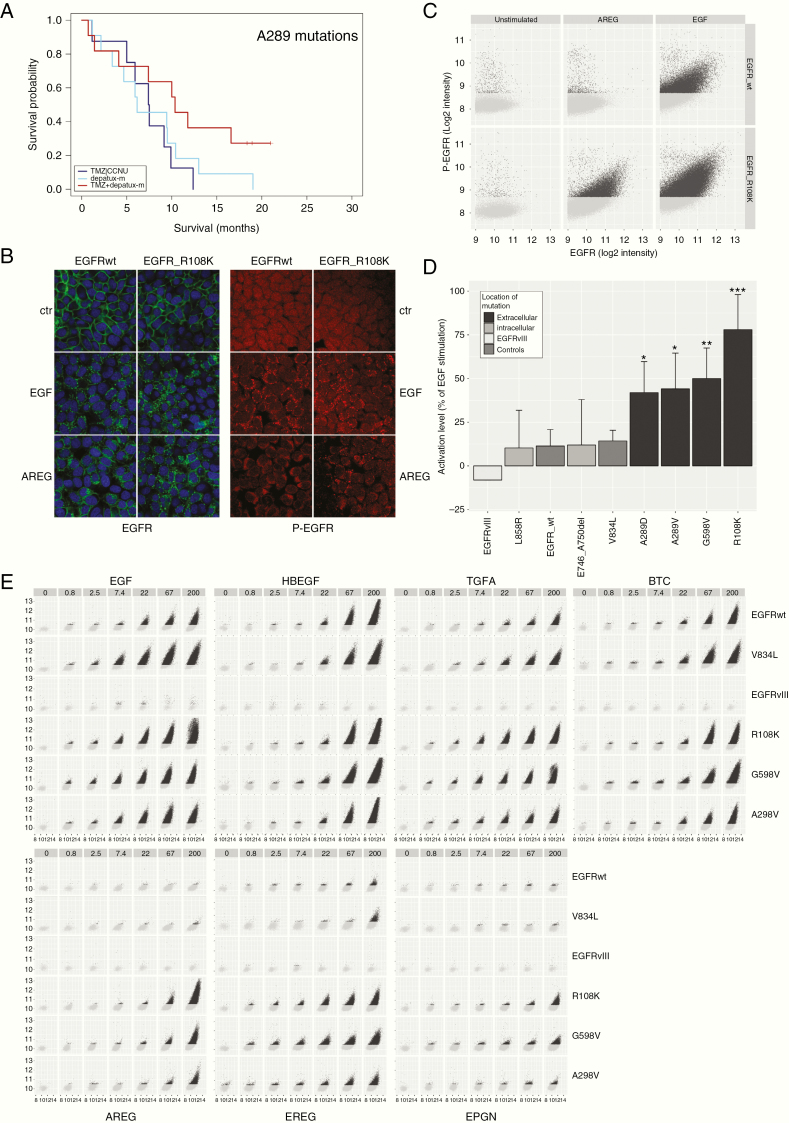
Figure 4.
(A) A289x hotspot mutations are associated with response to depatux-m + TMZ. (B) Example of microscopic images of EGFR-wt and EGFRR108K stimulated with either EGF or AREG. Green: EGFR, red: phospho-EGFR, blue: Hoechst. AREG resulted in increased receptor endocytosis and phospho-EGFR signal in EGFRR108K compared to EGFR-wt. (C) Example of analysis depicting the level of phospho-EGFR (y-axis) against total EGFR (x-axis) within individual EGFR positive vesicles. Each dot represents one EGFR-positive submenbranous vesicle. (D) Averages of experiments plotted in (C). (E) Similar to (B) the level of phospho-EGFR (y-axis) is plotted against total EGFR (x-axis) within individual EGFR positive vesicles. As can be seen, apart from EGFRvIII, all constructs responded, dose dependently, to the high affinity ligands EGF, HB-EGF, TGFA, and BTC by an increase in EGFR phosphorylation. Low affinity ligands AREG, EREG, and EPGN were not able to stimulate EGFR-wt constructs, but resulted in a strong activation of extracellular domain mutations EGFRR108K, EGFRA289V, and EGFRG598V. The absence of responses in EGFRvIII-expressing cells is in line with the notion that this mutation is independent of ligand. Images were taken at 40× magnification.