Abstract
Background
Human cytomegalovirus (HCMV) is an oncomodulatory human herpesvirus that has been detected in glioblastoma (GBM) and is associated with worse prognosis in patients with the disease. The effects of HCMV systemic infection on survival in GBM patients, however, are largely unknown. We aimed to determine the association between HCMV serostatus at diagnosis and survival via a retrospective cohort study of GBM patients.
Methods
Plasma from 188 GBM patients treated at the Ben and Catherine Ivy Center (Seattle, WA) was tested for HCMV serostatus via enzyme-linked immunosorbent assays of anti-HCMV immunoglobulin (Ig)G. HCMV IgG serostatus was analyzed with respect to each patient’s progression-free and overall survival (OS) via log-rank and multivariable Cox regression analysis.
Results
Ninety-seven of 188 (52%) patients were anti-HCMV IgG seropositive. Median OS was decreased in the IgG+ cohort (404 days) compared to IgG− patients (530 days; P = .0271). Among O6-methylguanine-DNA methyltransferase (MGMT) unmethylated patients (n = 96), median OS was significantly decreased in IgG+ patients (336 days) compared to IgG− patients (510 days; P = .0094). MGMT methylation was associated with improved OS in IgG+ patients versus those who were unmethylated (680 vs 336 days; P = .0096), whereas no such association was observed among IgG− patients.
Conclusions
In this study, HCMV seropositivity was significantly associated with poorer OS in GBM patients. This finding suggests prior infection with HCMV may play an important role in GBM patient outcomes, and anti-HCMV antibodies may, therefore, prove a valuable prognostic tool in the management of GBM patients.
Keywords: antibodies, glioblastoma, human cytomegalovirus, serostatus, survival
Key Points.
Cytomegalovirus in glioblastoma (GBM) tumors has been associated with worse prognosis.
HCMV seropositivity is associated with poorer OS in GBM patients.
First study to establish association between HCMV serostatus and OS in GBM patients.
Importance of the Study.
Human cytomegalovirus (HCMV) is a known oncomodulatory agent that increases malignancy and stemness in glioblastoma (GBM), and higher levels of HCMV infection in GBM tumors are associated with poorer prognosis. In addition, antivirals and anti-HCMV immunotherapy approaches have demonstrated early evidence of improved outcomes in GBM patients.
Our study shows for the first time that HCMV IgG seropositivity is associated with decreased overall survival in GBM patients. These findings have significant implications for GBM screening and treatment, given the potential utility of anti-HCMV IgG testing as a prognostic tool. The results of this study also indicate the need for further study of antiviral agents and virus-targeted immunotherapy in the treatment of GBM.
Glioblastoma (GBM), a World Health Organization Grade IV astrocytoma, is the most common and aggressive malignancy of the central nervous system. Despite advances in the field, the disease remains incurable with a median survival of just 14.6 months under standard-of-care treatment comprising surgical resection, radiotherapy, and concomitant and adjuvant chemotherapy with the DNA alkylating agent temozolomide.1 One of the few known prognostic indicators of overall survival (OS) in GBM patients is O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status. MGMT promoter methylation silences the expression of AGT, a DNA repair protein that counteracts temozolomide’s DNA alkylation of tumor cells by removing alkyl adducts from guanine bases.2 Suppression of AGT expression via MGMT methylation is thereby beneficial to OS in GBM patients.3
Human cytomegalovirus (HCMV), a betaherpesvirus that infects a majority of the US population,4 was first detected in the GBM tumor cells of immunocompetent patients by Cobbs et al.5 Following infection with HCMV, the virus persists in the host with long periods of quiescence and periodic reactivation. During initial infection and reactivation, the body rapidly produces anti-HCMV immunoglobulin (Ig)M antibodies while IgG levels are gradually built up to suppress the effects of chronic infection. As a result of HCMV infection, serious disease can develop in the immunocompromised (eg, neonates and AIDS patients), and though most healthy people infected with HCMV have no clinically significant symptoms, persistent infection can result in T-cell exhaustion and chronic, “smoldering” inflammation—two established oncomodulatory factors.6 Several HCMV antigens have also been shown to exacerbate hallmarks of tumor progression.7–13
HCMV DNA, RNA, and proteins have all been detected in GBM tumor tissue,5,14–18 while some studies have failed to detect the virus in GBM using standard laboratory techniques.19,20 Given the known oncomodulatory effects of HCMV infection, early successes of antiviral drugs and HCMV-targeting immunotherapy in improving the prognosis for GBM patients, and recent findings that HCMV seropositive GBM patients undergoing radio(chemo)therapy are predisposed to viral reactivation-linked encephalopathy,21 we hypothesized that systemic infection with HCMV may negatively affect survival in GBM patients. Here we present a retrospective cohort study analyzing the HCMV serostatus of 188 GBM patients and their overall and progression-free survival (PFS).
Materials and Methods
Study Population
The study was approved by the appropriate institutional review board. All appropriately consented Swedish Medical Center patients who had undergone surgery for primary GBM resection or biopsy over a period of approximately 10 years, with adequate available plasma from blood collected perioperatively for use in Ivy Center research studies (n = 188), were included in this study. Two patients did not have sufficient available plasma for testing and were excluded from the study. Each patient’s date of death was established using the date listed in the electronic health record (EHR) or, when no such date was recorded, using at least 2 cross-referenced sources: state death records, private genealogy database results (Genealogy Bank, Ancestry.com, Social Security Death Index), and online obituaries. Entries from these sources were matched to each patient using name and date of birth. Patients without confirmed date of death or date of tumor progression were censored from analyses of OS (at last known alive date) and PFS (at date of last known imaging without evidence of progression or, for patients without such imaging, the last known clinic visit), respectively.
Study Procedures
Peripheral blood samples were collected during initial resection or biopsy of each patient’s tumor, from which plasma was aspirated and subsequently stored for analysis. Plasma samples were analyzed for anti-HCMV IgG by enzyme-linked immunosorbent assay (ELISA). Imaging was obtained via magnetic resonance (MR) during normal diagnosis and follow-up according to each patient’s treating physicians. For patients who were not eligible for MR, such as those with pacemakers, computed tomography (CT) imaging was used. Disease progression was determined according to oncologist assessment in the EHR or, when such assessments were unavailable, the earlier of at least 2 consecutive MR/CT images assessed by the interpreting radiologist to show evidence of progression. The requirement for 2 consecutive images was implemented to rule out pseudoprogression commonly associated with radiation treatment. Pathological examinations of the patients’ tumors were gathered from the EHR and were originally conducted as part of each patient’s clinical treatment by CellNetix Pathology & Laboratories, LLC.
Laboratory Methods
Human blood samples
All patient blood samples were collected after informed consent was obtained for use of patient samples and data in research. At the time of each patient’s surgery, whole blood was collected into ethylenediaminetetraacetic acid vacutainer tubes and subsequently inverted 8–10 times. Blood samples were processed within 1 hour of collection. Tubes were centrifuged at 1300g for 10 minutes at room temperature, after which plasma was carefully aspirated and promptly stored at −80°C until use in the following assay.
Enzyme-linked immunosorbent assay
HCMV serostatus was determined in batches using an HCMV IgG ELISA kit according to the manufacturer’s instructions. All samples and controls were assayed in duplicate. Samples with equivocal results (cutoff ± 10%) or high deviation between replicates (optical density ratio difference >0.05) were assayed again. The HCMV qualitative ELISA kit for IgG (ab108724) was from Abcam PLC.
Statistical Analysis
Data were summarized using frequencies and percentages. Patient characteristics were analyzed with respect to HCMV IgG serostatus using χ 2 or Fisher’s exact tests. Survival times were described using medians. Survival curves were constructed for OS and PFS using Kaplan–Meier estimates and compared using the log-rank test. Cox regression method was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for comparison of the 2 serostatus groups adjusting for age, sex, extent of resection, and radio(chemo)therapy. All analyses were conducted using SAS version 9.4 (SAS Institute, Inc). Significance was considered at P values < .05.
Results
A total of 188 patients were included in the initial cohort for analysis (Table 1), and multivariable Cox regression analysis was performed for the entire cohort (Table 2), as well as for those with known MGMT methylation status (Table 3). Patients with unknown temozolomide or radiation treatment status were omitted from both Cox regression analyses. Extent of resection was categorized as biopsy-only or partial/complete resection for analysis purposes.
Table 1.
Patient characteristics (n = 188)
| Characteristic | N (%) | IgG− (%) | IgG+ (%) | P Value |
|---|---|---|---|---|
| Age at first surgery | .0850 | |||
| <50 | 34 (18) | 21 (23) | 13 (13) | |
| ≥50 | 154 (82) | 70 (77) | 84 (87) | |
| Sex | .7060 | |||
| Male | 111 (59) | 55 (60) | 56 (58) | |
| Female | 77 (41) | 36 (40) | 41 (42) | |
| Race | .5100 | |||
| White/Caucasian | 152 (81) | 78 (86) | 74 (76) | |
| Asian | 7 (4) | 2 (2) | 5 (5) | |
| Black/African American | 2 (1) | 1 (1) | 1 (1) | |
| American Indian/Alaska Native | 2 (1) | 1 (1) | 1 (1) | |
| Other/no response | 25 (13) | 9 (10) | 16 (16) | |
| Extent of resection | .8321a | |||
| None (biopsy) | 5 (3) | 2 (2) | 3 (3) | |
| Partial | 104 (55) | 49 (54) | 55 (57) | |
| Complete | 79 (42) | 40 (44) | 39 (40) | |
| MGMT methylation (n = 141) | .2817 | |||
| Unmethylated | 96 (68) | 44 (64) | 52 (72) | |
| Methylated | 45 (32) | 25 (36) | 20 (28) | |
| Temozolomide chemotherapy (n = 165) | .2829a | |||
| Not received | 8 (5) | 2 (3) | 6 (7) | |
| Received | 157 (95) | 76 (97) | 81 (93) | |
| Radiotherapy (n = 164) | .3690a | |||
| Not received | 5 (3) | 1 (1) | 4 (5) | |
| Received | 159 (97) | 78 (99) | 81 (95) |
Each characteristic’s association with HCMV IgG serostatus analyzed using χ 2 test unless noted. HCMV, human cytomegalovirus; MGMT, O6-methylguanine-DNA methyltransferase.
aAssociation with HCMV IgG serostatus analyzed using Fisher’s exact test.
Table 2.
Multivariable Cox regression analysis for overall survival, patients with known status of temozolomide (TMZ), and radiation treatment (n = 161)
| Factor | HR | 95% CI | P Value |
|---|---|---|---|
| Age (unit = 5) | 1.07 | 0.99–1.16 | .0936 |
| Male sex (vs female) | 1.23 | 0.88–1.74 | .2319 |
| IgG+ (vs IgG−) | 1.47 | 1.02–2.10 | .0370 |
| Complete/partial resection (vs biopsy-only) | 0.19 | 0.07–0.52 | .0015 |
| TMZ treatment (vs none) | 0.35 | 0.12–1.04 | .0597 |
| Radiation treatment (vs none) | 0.41 | 0.10–1.60 | .1985 |
CI, confidence interval; HR, hazard ratio.
Table 3.
Multivariable Cox regression analysis for overall survival, patients with known status of MGMT methylation, and temozolomide (TMZ) and radiation treatment (n = 124)
| Factor | HR | 95% CI | P Value |
|---|---|---|---|
| Age (unit = 5) | 1.04 | 0.95–1.15 | .4123 |
| Male sex (vs female) | 1.28 | 0.86–1.91 | .2313 |
| IgG+ (vs IgG−) | 1.71 | 1.13–2.59 | .0120 |
| Complete/partial resection (vs biopsy-only) | 0.19 | 0.06–0.64 | .0071 |
| MGMT methylated (vs unmethylated) | 0.48 | 0.31–0.76 | .0014 |
| TMZ treatment (vs none) | 0.30 | 0.09–0.99 | .0482 |
| Radiation treatment (vs none) | 0.55 | 0.09–3.55 | .5324 |
CI, confidence interval; HR, hazard ratio; MGMT, O6-methylguanine-DNA methyltransferase.
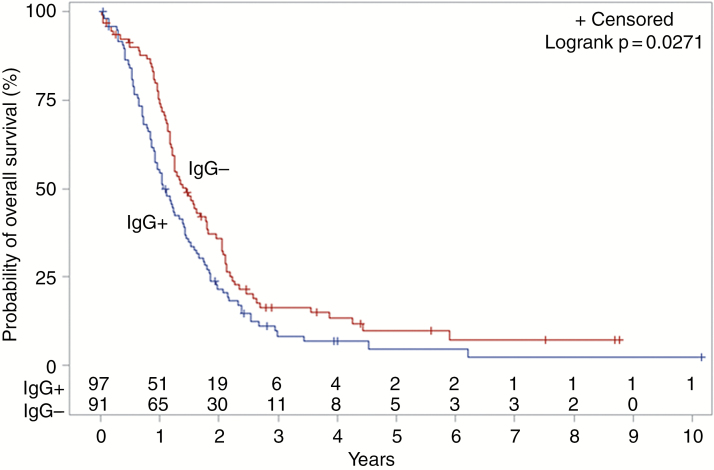
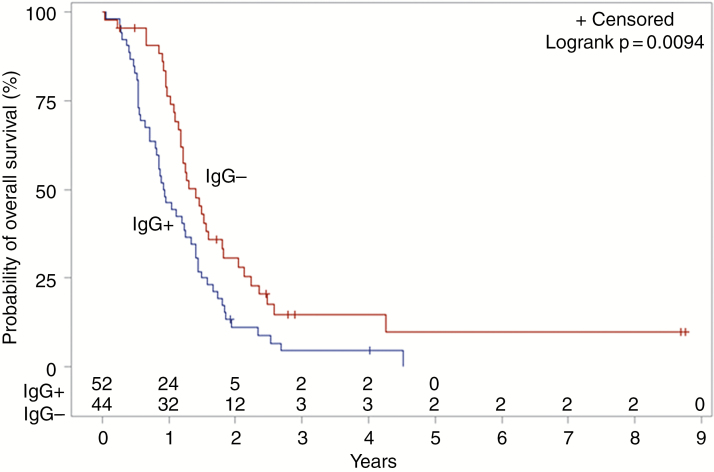
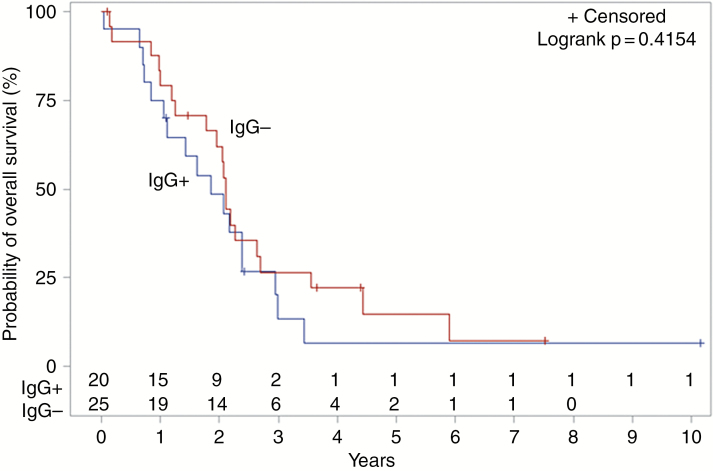
Ninety-seven of 188 (51.6%) patients tested positive for anti-HCMV IgG antibodies. Log-rank testing showed an association between anti-HCMV IgG serostatus and OS, with median OS decreased in the IgG+ cohort (404 days) compared to IgG− (530 days; P = .0271; Fig. 1). After adjusting for age, sex, extent of resection, and radio(chemo)therapy, the negative impact of seropositivity with OS persisted (HR 1.47; CI 1.02–2.10; P = .0370; Table 2). This finding was consistent for patients with known MGMT methylation status, for whom the negative impact of IgG seropositivity on OS persisted after adjusting for the same variables (HR 1.71; CI 1.13–2.59; P = .0120; Table 3). Among MGMT unmethylated patients (n = 96), median OS was significantly decreased in IgG+ patients (336 days) compared to IgG− patients (510 days; P = .0094; Fig. 2). No difference in median OS was observed between IgG+ and IgG− patients known to be MGMT methylated (n = 45; P = .4154; Fig. 3). MGMT methylation was associated with a significant improvement in median OS in anti-HCMV seropositive patients (680 days for MGMT methylated patients versus 336 days for unmethylated patients; P = .0096), but there was no significant difference in median OS for methylated compared to unmethylated patients among those who were seronegative (773 days vs 510 days; P = .1348). There was no correlation between IgG serostatus and PFS by any analysis.
Fig. 1.
Kaplan–Meier estimates of overall survival according to HCMV IgG serostatus (n = 188). HCMV, human cytomegalovirus.
Fig. 2.
Kaplan–Meier estimates of overall survival according to HCMV IgG serostatus, among MGMT unmethylated patients (n = 96). HCMV, human cytomegalovirus; MGMT, O6-methylguanine-DNA methyltransferase.
Fig. 3.
Kaplan–Meier estimates of overall survival according to HCMV IgG serostatus, among MGMT methylated patients (n = 45). HCMV, human cytomegalovirus; MGMT, O6-methylguanine-DNA methyltransferase.
For those patients with a verified date of death (n = 164), the median life span was 61.8 years. The median PFS for those patients demonstrating radiographic evidence of progression after initial surgery (n = 177) was 7.6 months.
Discussion
Despite evidence of correlation between high levels of HCMV protein expression in GBM tumor cells and poorer prognosis,17 as well as initial evidence that antiviral therapies may be effective in the treatment of GBM, the presence of HCMV in GBM has been the subject of intense controversy for several years. Our study demonstrates for the first time a correlation between prior systemic HCMV infection and decreased OS in GBM patients, a finding with high clinical relevance. This correlation was strongest among MGMT unmethylated patients (Fig. 2) and was not evident among those patients with MGMT methylation (Fig. 3), likely a consequence of the smaller subgroup of methylated patients in our study.
Of note, MGMT methylation—known to correlate with improved survival in GBM patients generally—was in this study significantly associated with improved OS for those patients who were anti-HCMV seropositive but was only suggestive of improved OS in those who were seronegative. There is no currently known link between MGMT methylation and HCMV serostatus on OS in GBM patients aside from the potentially additive deleterious effects of unmethylated status and seropositivity. Larger studies as well as mechanistic investigations are, therefore, warranted to elucidate any potential interactions between these characteristics.
Obtaining HCMV serology at diagnosis effectively rules out any treatment-related effects on serostatus and is commonly performed to examine the overall impact of the virus in various clinical contexts. There are several studies in the solid organ and hematopoietic cell transplant setting that have shown a negative impact of HCMV using this approach, even with the use of preemptive therapy.22,23 The effect size of the mortality difference in this study appears to be in the range that was previously shown in these transplant studies. These data provide further rationale for investigations into the possible mechanisms of HCMV oncomodulation.
A recent study by Goerig et al.21 demonstrated that HCMV can be reactivated during radio(chemo)therapy, leading to viremia and additional sequelae including potentially fatal encephalitis. For this reason, that study’s authors suggested that patients with brain tumors receiving radiation and steroids be screened for evidence of anti-HCMV IgG, followed by HCMV DNA screening in IgG+ patients, a recommendation shared by other groups in the context of solid tumor patients presenting with unexplained fever.24 Owing to the prognostic value of readily detectable circulating anti-HCMV IgG antibodies and the potential utility of HCMV serostatus as an indicator of patients who may need further interventions during treatment (eg, antivirals), our study provides the rationale to examine whether routine HCMV IgG serology testing for all patients with high-grade gliomas may be beneficial.
Antiviral agents and immunotherapy targeting viral antigens have both shown early promise in improving the prognosis of GBM patients. Valganciclovir, an antiviral that inhibits HCMV replication, has shown initial evidence for improving outcomes in GBM patients in an early phase study.25,26 Cidofovir, too, has shown effectiveness in killing GBM cells in vitro and in mice, though this effect is likely due to nonspecific DNA damage preferentially killing rapidly dividing tumor cells rather than specific interaction with the virus.27 HCMV epitope-targeting immunotherapy has been shown to lyse GBM cells in vitro28 as well as increase GBM patient overall and PFS in the case of one clinical trial.29 In addition, infusion of T cells stimulated ex vivo with HCMV epitopes has been shown to reduce GBM findings by MR imaging30 and improve post-recurrence OS.31 Notwithstanding the conflicting accounts of HCMV’s presence within GBM tumors, the results of this study clearly indicate the importance of validation in additional cohorts as well as continued exploration into virus-targeting immunotherapy and prophylactic antiviral treatment as adjuncts to the current standard-of-care regimen for GBM.
Limitations of this study include a limited sample size and single clinical site from which patients were drawn. These drawbacks are partially mitigated by the fact that this is one of the larger studies investigating cytomegalovirus in GBM patients and that our cohort is largely reflective of GBM patients nationally. Although all members of our study came from a single clinical site, the group appears to be highly representative of GBM patients in the United States. Our cohort had a 60.5 year median age at initial operation (vs 64 years nationally); 14.9 month median OS (vs 14.6 months1); 59% (111/188) male prevalence (vs 61%32); 51.6% (97/188) HCMV IgG seroprevalence (vs 50.4%4); and, for the 141 patients with MGMT promoter methylation status documented in the EHR, an association between methylation and better OS1 via log-rank testing (P = .0023). Further limitations include the possibility of unknown confounding variables, the potential for certain HCMV-infected patients to not have detectable anti-HCMV IgG antibodies either because they did not mount a significant immune response to infection or have undergone immune senescence, as well as the present lack of an identified mechanism connecting MGMT methylation status with anti-HCMV serostatus vis-à-vis survival in GBM patients. Although this study does not attempt to elucidate the causal links between HCMV infection and poorer prognosis, prior research suggests that contributing factors to the observed correlation may include some combination of direct HCMV-mediated oncomodulation as well as virus-induced local and systemic inflammation and/or immunosuppression.
In conclusion, we show here that HCMV seropositivity is associated with poorer OS in GBM patients, especially among those who are MGMT unmethylated. Further research is needed to examine the mechanisms of HCMV’s deleterious effects in the context of GBM and, ultimately, randomized controlled trials are warranted to examine the potential survival benefit of HCMV suppression in GBM patients.
Funding
The Ben and Catherine Ivy Foundation.
Conflict of interest statement. M.B. has received research funding and consulting fees from Merck, Chimerix, Inc, Takeda (formerly Shire), Astellas, and Vir Bio; and consulting fees from Helocyte and Artemis Therapeutics. All other authors have no conflicts of interest to declare.
Authorship statement. Experiment design: H.F. and C.C.; Collection and preparation of patient samples: Y.J.-G.; ELISA assays: H.F, K.P., and L.D.; Collection of clinical data: H.F., K.P., and L.D.; Data analysis and interpretation: H.F., H.-F.L., J.S., M.B., and C.C.; Writing and correction of manuscript: H.F., M.B., K.P., and C.C.; Figure creation: H.F. and H.-F.L.; Responsibility for accuracy of data analysis: H.-F.L. and J.S.
Acknowledgments
We wish to thank Hwahyung Lee and John Henson for their contributions to the study.
References
- 1. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. Gerson SL. Clinical relevance of MGMT in the treatment of cancer. J Clin Oncol. 2002;20(9):2388–2399. [DOI] [PubMed] [Google Scholar]
- 3. Hegi ME, Diserens AC, Godard S, et al. . Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004;10(6):1871–1874. [DOI] [PubMed] [Google Scholar]
- 4. Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis. 2010;50(11):1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cobbs CS, Harkins L, Samanta M, et al. . Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62(12):3347–3350. [PubMed] [Google Scholar]
- 6. Foster H, Ulasov IV, Cobbs CS. Human cytomegalovirus-mediated immunomodulation: effects on glioblastoma progression. Biochim Biophys Acta Rev Cancer. 2017;1868(1):273–276. [DOI] [PubMed] [Google Scholar]
- 7. Cobbs CS, Soroceanu L, Denham S, Zhang W, Kraus MH. Modulation of oncogenic phenotype in human glioma cells by cytomegalovirus IE1-mediated mitogenicity. Cancer Res. 2008;68(3):724–730. [DOI] [PubMed] [Google Scholar]
- 8. Lee K, Jeon K, Kim JM, et al. . Downregulation of GFAP, TSP-1, and p53 in human glioblastoma cell line, U373MG, by IE1 protein from human cytomegalovirus. Glia. 2005;51(1):1–12. [DOI] [PubMed] [Google Scholar]
- 9. Soroceanu L, Matlaf L, Khan S, et al. . Cytomegalovirus immediate-early proteins promote stemness properties in glioblastoma. Cancer Res. 2015;75(15):3065–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matlaf LA, Harkins LE, Bezrookove V, Cobbs CS, Soroceanu L. Cytomegalovirus pp71 protein is expressed in human glioblastoma and promotes pro-angiogenic signaling by activation of stem cell factor. PLos One. 2013;8(7):e68176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soroceanu L, Matlaf L, Bezrookove V, et al. . Human cytomegalovirus US28 found in glioblastoma promotes an invasive and angiogenic phenotype. Cancer Res. 2011;71(21):6643–6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ulasov IV, Kaverina NV, Ghosh D, et al. . CMV70-3P miRNA contributes to the CMV mediated glioma stemness and represents a target for glioma experimental therapy. Oncotarget. 2017;8(16):25989–25999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cobbs C, Khan S, Matlaf L, et al. . HCMV glycoprotein B is expressed in primary glioblastomas and enhances growth and invasiveness via PDGFR-alpha activation. Oncotarget. 2014;5(4):1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lucas KG, Bao L, Bruggeman R, Dunham K, Specht C. The detection of CMV pp65 and IE1 in glioblastoma multiforme. J Neurooncol. 2011;103(2):231–238. [DOI] [PubMed] [Google Scholar]
- 15. Rahbar A, Stragliotto G, Orrego A, et al. . Low levels of human cytomegalovirus infection in glioblastoma multiforme associates with patient survival; -a case-control study. Herpesviridae. 2012;3(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scheurer ME, Bondy ML, Aldape KD, Albrecht T, El-Zein R. Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathol. 2008;116(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rahbar A, Orrego A, Peredo I, et al. . Human cytomegalovirus infection levels in glioblastoma multiforme are of prognostic value for survival. J Clin Virol. 2013;57(1):36–42. [DOI] [PubMed] [Google Scholar]
- 18. Mitchell DA, Xie W, Schmittling R, et al. . Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol. 2008;10(1):10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holdhoff M, Guner G, Rodriguez FJ, et al. . Absence of cytomegalovirus in glioblastoma and other high-grade gliomas by real-time PCR, immunohistochemistry, and in situ hybridization. Clin Cancer Res. 2017;23(12):3150–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baumgarten P, Michaelis M, Rothweiler F, et al. . Human cytomegalovirus infection in tumor cells of the nervous system is not detectable with standardized pathologico-virological diagnostics. Neuro Oncol. 2014;16(11):1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goerig NL, Frey B, Korn K, et al. . Frequent occurrence of therapeutically reversible CMV-associated encephalopathy during radiotherapy of the brain. Neuro Oncol. 2016;18(12):1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boeckh M, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004;103(6):2003–2008. [DOI] [PubMed] [Google Scholar]
- 23. Teira P, Battiwalla M, Ramanathan M, et al. . Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schlick K, Grundbichler M, Auberger J, et al. . Cytomegalovirus reactivation and its clinical impact in patients with solid tumors. Infect Agent Cancer. 2015;10(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stragliotto G, Rahbar A, Solberg NW, et al. . Effects of valganciclovir as an add-on therapy in patients with cytomegalovirus-positive glioblastoma: a randomized, double-blind, hypothesis-generating study. Int J Cancer. 2013;133(5):1204–1213. [DOI] [PubMed] [Google Scholar]
- 26. Söderberg-Naucler C, Peredo I, Rahbar A, Hansson F, Nordlund A, Stragliotto G. Use of Cox regression with treatment status as a time-dependent covariate to re-analyze survival benefit excludes immortal time bias effect in patients with glioblastoma who received prolonged adjuvant treatment with valganciclovir. Int J Cancer. 2014;135(1):248–249. [DOI] [PubMed] [Google Scholar]
- 27. Hadaczek P, Ozawa T, Soroceanu L, et al. . Cidofovir: a novel antitumor agent for glioblastoma. Clin Cancer Res. 2013;19(23):6473–6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghazi A, Ashoori A, Hanley PJ, et al. . Generation of polyclonal CMV-specific T cells for the adoptive immunotherapy of glioblastoma. J Immunother. 2012;35(2):159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Batich KA, Reap EA, Archer GE, et al. . Long-term survival in glioblastoma with cytomegalovirus pp65-targeted vaccination. Clin Cancer Res. 2017;23(8):1898–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crough T, Beagley L, Smith C, Jones L, Walker DG, Khanna R. Ex vivo functional analysis, expansion and adoptive transfer of cytomegalovirus-specific T-cells in patients with glioblastoma multiforme. Immunol Cell Biol. 2012;90(9):872–880. [DOI] [PubMed] [Google Scholar]
- 31. Schuessler A, Smith C, Beagley L, et al. . Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer Res. 2014;74(13):3466–3476. [DOI] [PubMed] [Google Scholar]
- 32. Ostrom QT, Gittleman H, Xu J, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 2016;18(suppl 5):v1–v75. [DOI] [PMC free article] [PubMed] [Google Scholar]