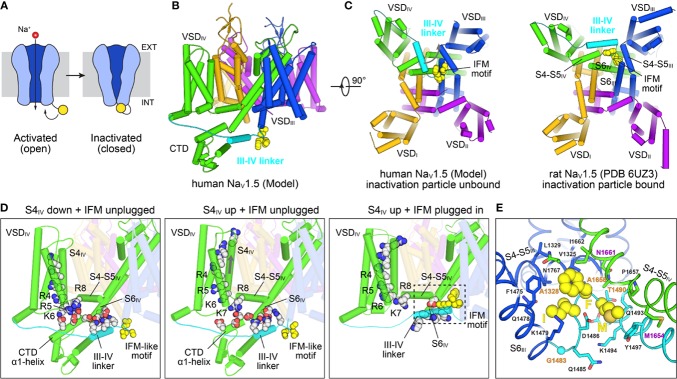
Figure 6.
Structural mechanisms of NaV channel inactivation. (A) NaV channels transition from an activated open state to a non-conducting inactivated state after depolarization. Inactivation is induced by binding of a C-terminal motif (yellow) to the cytosolic side of the channel leading to pore closure. The cell membrane is indicated with a gray rectangle, with the extracellular (EXT) and intracellular space (INT) labeled. (B) Closed state model of SCN5A (Kroncke et al., 2019) highlighting structural elements involved in fast inactivation. The III-IV linker (cyan) connecting S6III (blue) with VSDIV (green) contains the IFM motif (yellow spheres) which is the key structural element responsible for inactivation. The position of the III-IV linker is constrained by the CTD following S6IV. (C) Comparison of the SCN5A model in (B) with a cryo-EM structural model of rat SCN5A (PDB: 6UZ3)(Jiang et al., 2020) in a putative inactivated state viewed from the intracellular side. The III-IV linker undergoes a large shift in the SCN5A structure and the IFM motif is docked into a pocket surrounded by the S4–S5 linkers and S6 helices of repeats III and IV. (D) Structural states and transitions proposed to be involved in fast inactivation in NaV channels. Left: Structure of a chimeric NaV1.7–NaVPaS channel (Clairfeuille et al., 2019) (PDB: 6NT3) with S4IV in a “down” position and an IFM-like motif unbound. Basic sites R5–R8 bridge to conserved acidic residues on the CTD which facilitates binding of the III-IV linker to S6IV. Middle: Structure of a chimeric NaV1.7–NaVPaS channel (Clairfeuille et al., 2019) (PDB: 6NT4) with S4IV in a ‘up’ position and an IFM-like motif unbound. The electrostatic bridge between S4IV and the CTD is broken possibly increasing the positional dynamics of the CTD and III-IV linker. Right: Structure of SCN5A (PDB: 6UZ3) (Jiang et al., 2020) with S4IV in an ‘up’ position and the IFM motif plugged into the cytosolic cavity between repeats III and IV. Note, that the CTD is missing in the cryo-EM structure. (E) IFM binding pocket residues on S4–S5III, S4–S5IV, S6III, and S6IV of SCN5A (PDB: 6UZ3) (Jiang et al., 2020). The S6IV helix backbone in the front is not shown for clarity. Residues that are important for fast inactivation and those found in various types of myotonia (Pan et al., 2018) are labeled magenta and orange, respectively.