Figure 1.
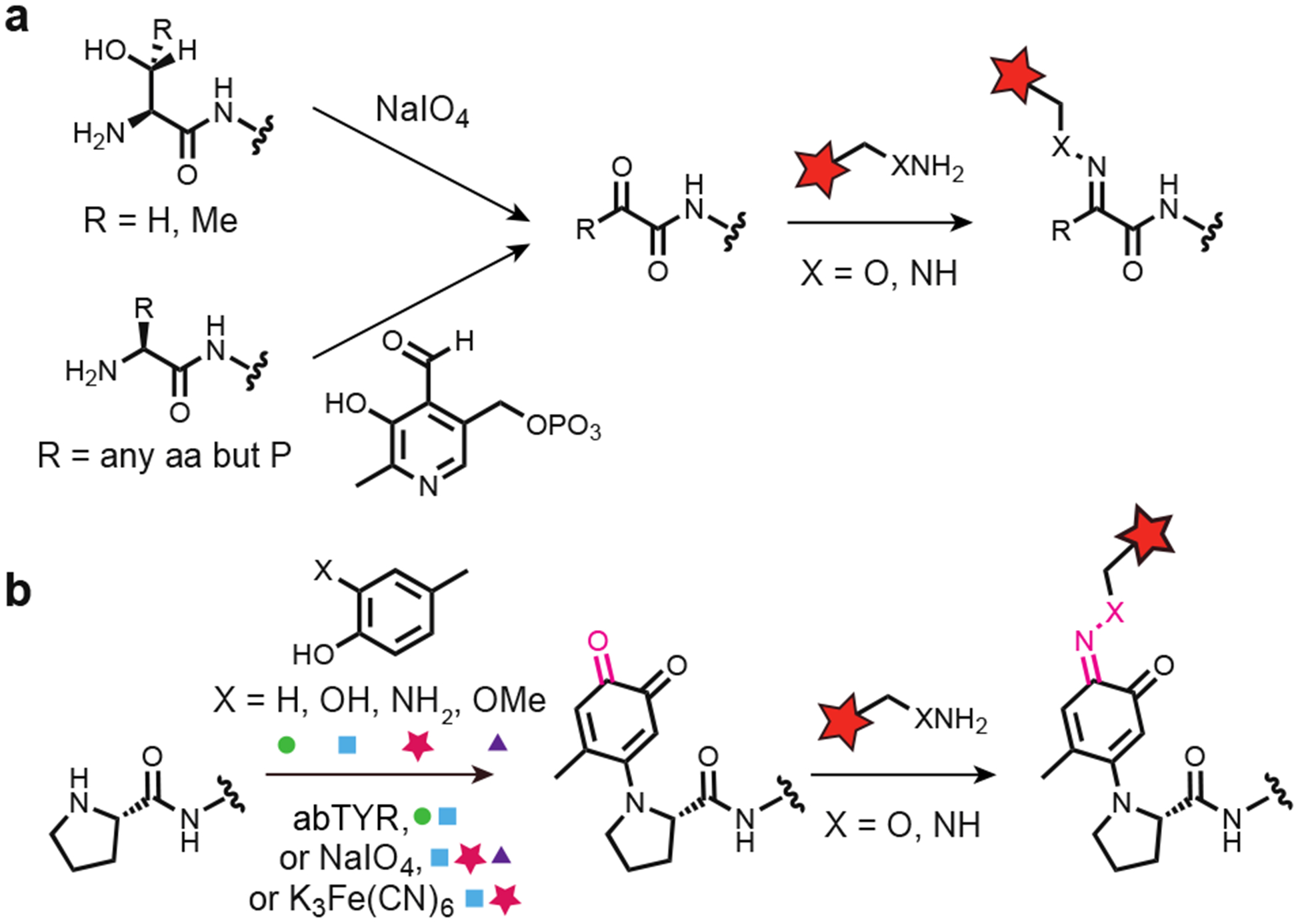
Chemoselective oxime and hydrazone formation for bioconjugation reactions using endogenous N-termini. (a) N-terminal ketones can be formed either via an oxidative cleavage of an N-terminal ser or thr using sodium periodate, or via a transamination reaction between the native N-terminus and PLP analogues. These reactive ketones readily undergo condensation reactions with either alkoxyamine- or hydrazine-bearing cargo, to form oxime and hydrazone linkages, respectively. (b) Reaction of a pro N-terminus via the oxidative coupling results in a product bearing a single ketone. This carbonyl could subsequently be reacted with an alkoxyamine or hydrazine, extending oxime and hydrazone formation to pro N-termini.
