Figure 3.
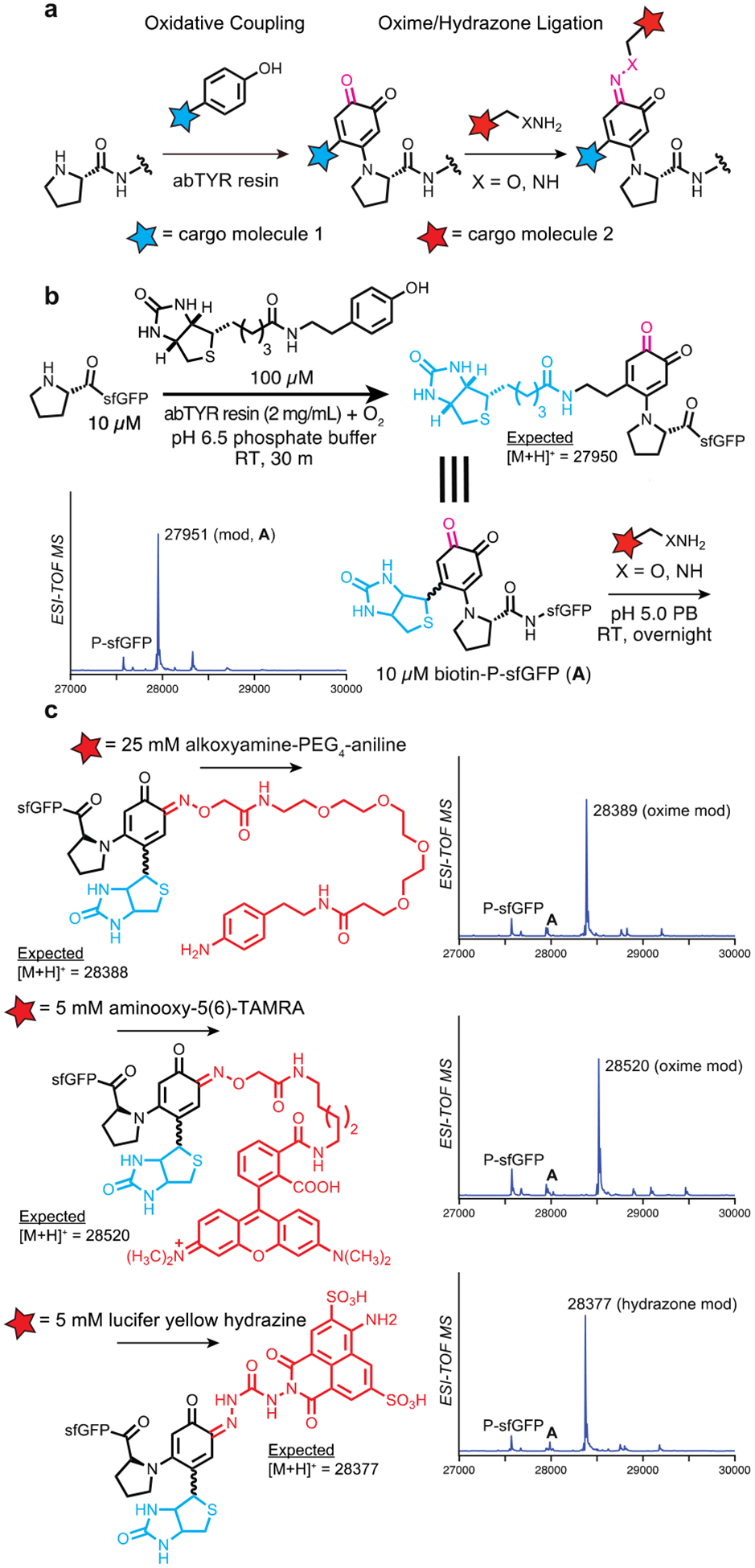
Construction of bifunctional proline N-terminal bioconjugates using a sequentional oxidative coupling and oxime/hydrazone ligation, (a) Using an initial oxidative coupling reaction, primary cargo can be installed at the proline N-terminus. Subsequent oxime or hydrazone ligation can then be used to install secondary cargo at the N-terminus using the oxidative coupling product, (b) Synthesis of N-terminally biotinylated pro-sfGFP A was carried out using abTYR resin and a biotin-phenol, installing both ketone and biotin moieties at the proline N-terminus. (c) Secondary cargo installation was performed on product A using an alkoxyamine-aniline, alkoxyamine-TAMRA, and a lucifer yellow hydrazine. All reagents afforded near-complete conversion to the expected products when incubated with A overnight.
