Figure 8.
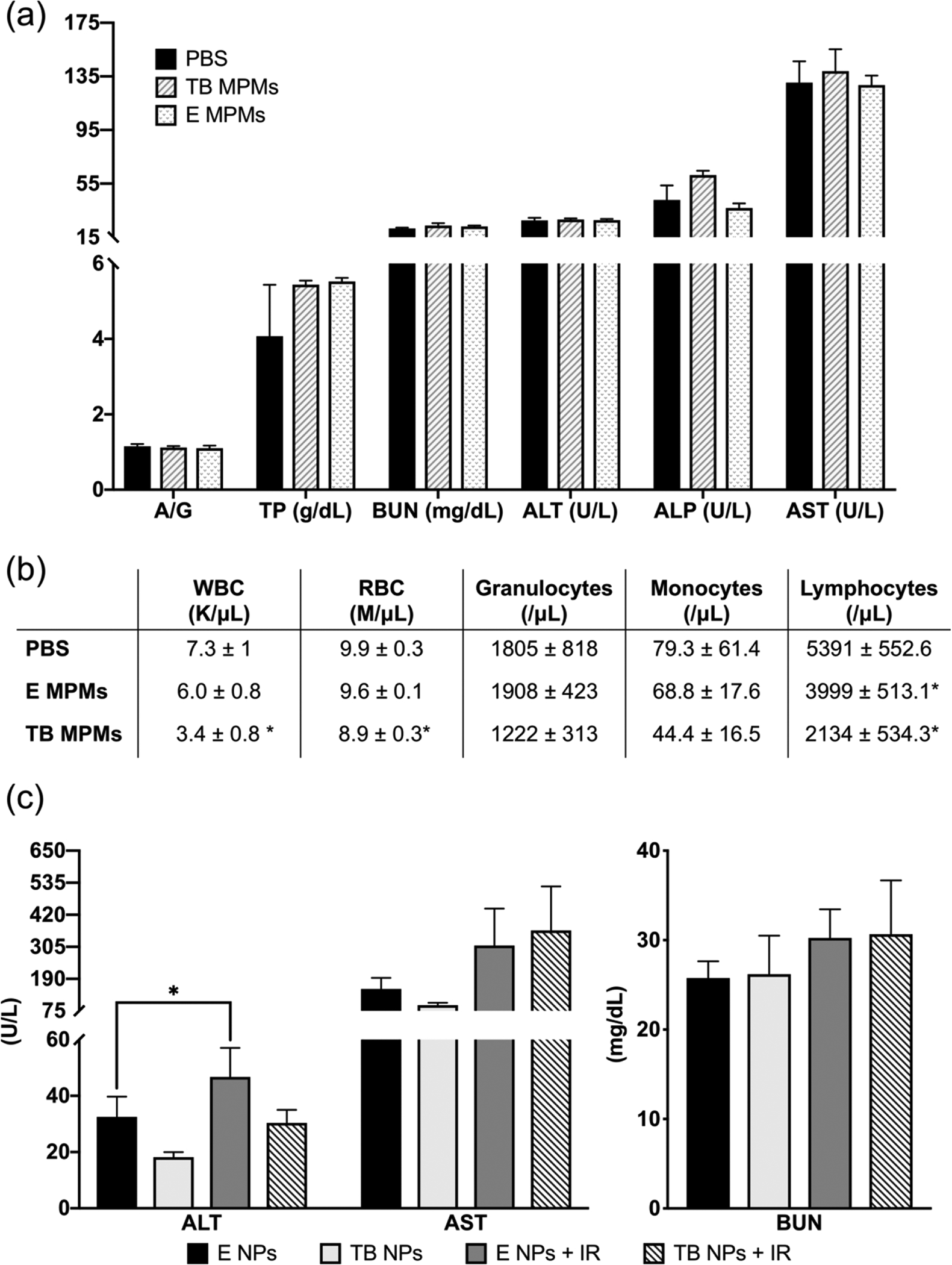
In vivo evaluation of acute toxicity of encapsulated drugs and carrier. (a) Plasma clinical chemistry markers of liver and kidney function and (b) Hematology toxicity parameters from healthy, tumor-free mice 96 hours after a single dose of PBS (control), E MPMs (310 mg/kg poloxamers), or TB MPMs (1.3 mg/kg tal, 1.23 mg/kg bup). (c) Plasma clinical chemistry markers of chronic toxicity of encapsulated drugs and carrier monitoring liver (ALT and AST) and kidney (BUN function.) * denotes statistically significant difference compared to PBS control (p < 0.05).
