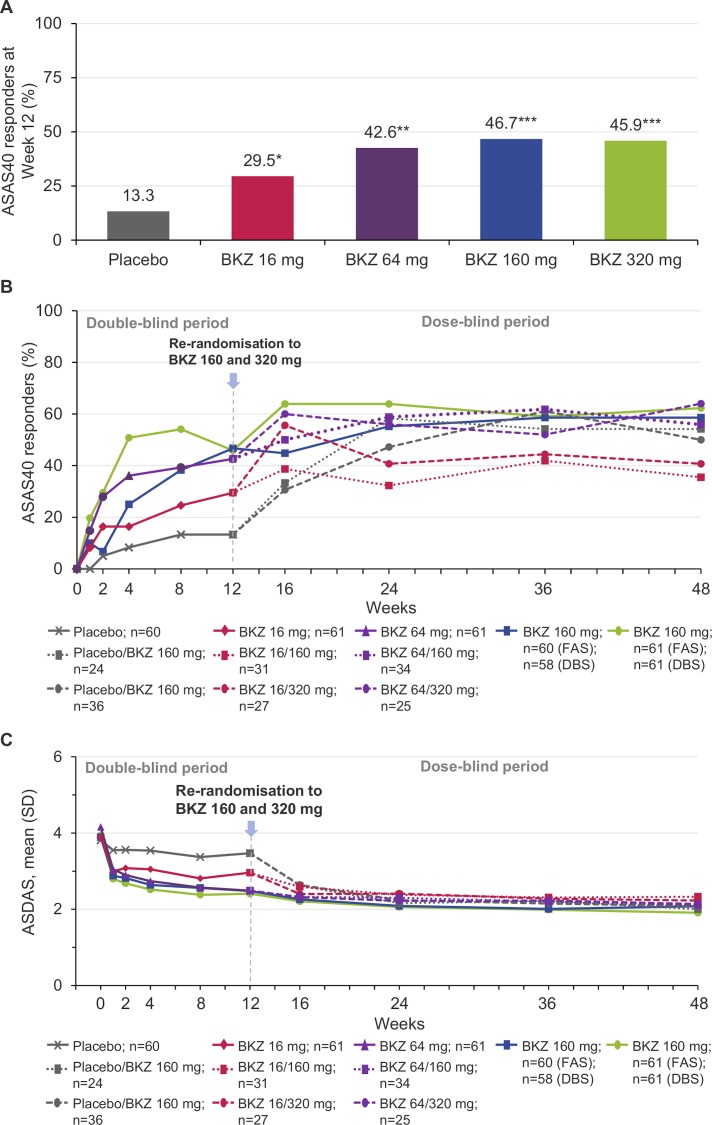
Figure 1.
(A) ASAS40 response at week 12 (primary efficacy end point; FAS, NRI). *P value vs placebo calculated from a logistic regression model including fixed effects for treatment, geographic region and prior TNF inhibitor exposure; *p<0.05, **p<0.01, ***p<0.001. (B) ASAS40 responses over 48 weeks; FAS, NRI (weeks 0–12); DBS, NRI (weeks 12–48). (C) ASDAS over time; FAS, MI (weeks 0–12); DBS, MI (weeks 12–48). ASAS40, Assessment of SpondyloArthritis international Society improvement of ≥40%; ASDAS, Ankylosing Spondylitis Disease Activity Score; BKZ, bimekizumab; DBS, dose-blind set; FAS, full analysis set; MI, multiple imputation; NRI, non-responder imputation.