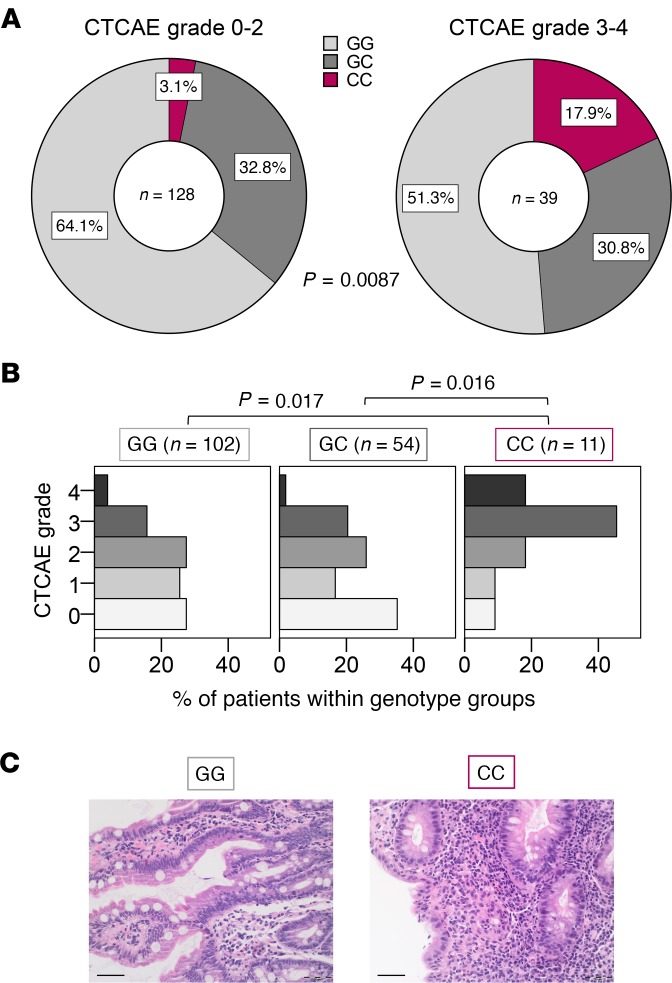
Figure 4. SNP rs2910164 in the MIR146A gene is associated with increased irAE severity in patients.
Genotype of the SNP rs2910164 in the MIR146A gene was determined from 167 patients with cancer treated with ≥3 cycles anti–PD-1 or anti–PD-L1 therapy. irAEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE). (A) Genotype frequencies for the group of patients who developed no/mild irAEs (CTCAE grades 0–2) or severe irAEs (CTCAE grades 3–4), respectively, are shown. Fisher’s exact test was used to analyze the contingency table. (B) Percentage of patients with CTCAE grades 0–4 irAEs in the different genotype groups is shown. Data were statistically analyzed by Kruskal-Wallis test followed by post hoc pairwise comparison Dunn’s test and P values were adjusted by Bonferroni’s correction. (C) Exemplary colon histopathology pictures of a GG patient with grade 1 intestinal irAEs (left) and of a CC patient with grade 4 intestinal irAEs (right) are shown. Scale bar: 50 μm. irAE, immune-related adverse event, PD-1, programmed cell death protein-1.