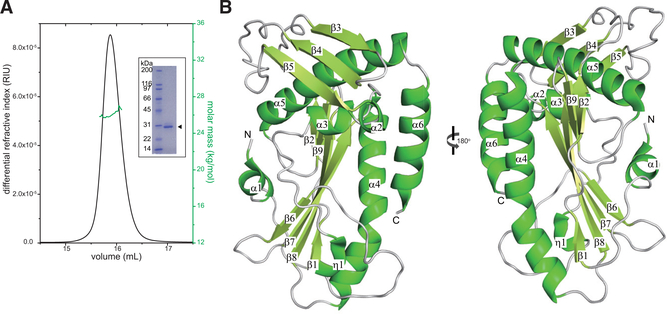
Figure 1. The SFTSV L Endonuclease Domain Is a Monomer in Solution and Adopts a Mixed Alpha-Beta Fold.
(A) SEC-MALS analysis of SFTSV L 1–231 aa. The determined mass of SFTSV L 1–231 aa (green) is 2.71 ± 0.01 × 104 Da. The theoretical mass for the monomer is 2.76 × 104 Da. Independent experiments were carried out in triplicate and representative data are shown here. Inset: Coomassie-stained SDS-PAGE of purified, recombinant N-terminal His-tagged SFTSV L endonuclease domain 1–231 aa (arrowhead).
(B) X-ray crystal structure of the SFTSV L endonuclease domain. Secondary structural elements that form the α helices, the β sheets, and the loop regions are annotated based on the relative position from the N terminus.