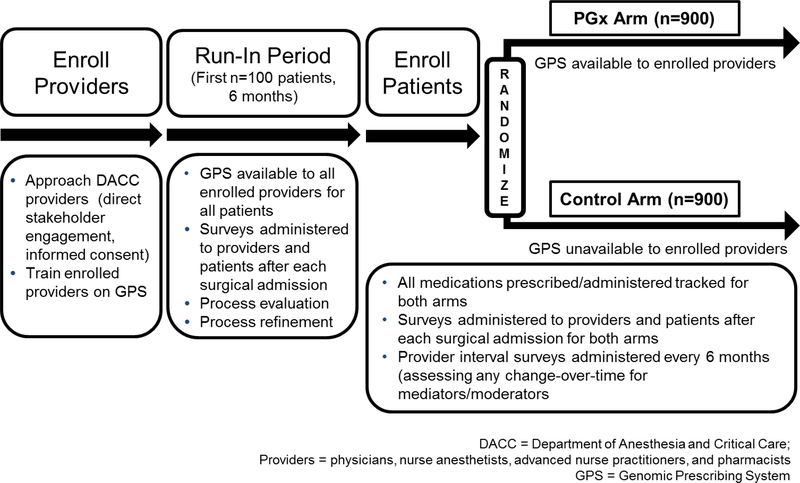
Figure 1.
Study schema for the The ImPreSS Trial: Implementation of Point-of-Care Decision Support in Perioperative Setting. The DACC has approximately 130 clinical personnel who will be targeted for participation, and we anticipate being able to successfully enroll 70% of these providers. Therefore, we expect to enroll approximately 90 providers and approximately 20 patients per provider during the randomization phase [n=1800 total patients who will be randomly assigned to the control (n=900) or the PGx arm (n=900)]. The randomization process will allow us to compare the frequency of GPS use by DACC providers in both arms and to determine the rate of use of high-risk drugs in the group of patients for whom pharmacogenomic results are available compared to their rate of use in the control arm (without provider knowledge of pharmacogenomic risk designation). We will use a mixed effects model and include provider as a random effect to allow for correlation of responses among patients treated by the same provider. All medications prescribed/administered will be tracked for both arms. Providers and patients will be asked to complete a survey after each surgical admission. Providers will also be asked to complete a questionnaire every six months thereafter to assess knowledge and attitudes towards pharmacogenomics. For the pain sub-analysis, we anticipate that 20% of the randomized patients will receive pain consultations after each surgery (n=180 in each arm). Pain will be assessed using a 11-point numeric rating scale, and differences between groups will be compared using a two-sample t-test. Assuming a pooled standard deviation of 2.5 (range of 10/4), the study sample size of 360 (n=180 for each group) will achieve 85% power and a level of significance of 5% (p<0.05) for detecting a true mean difference of 0.8 between the groups.