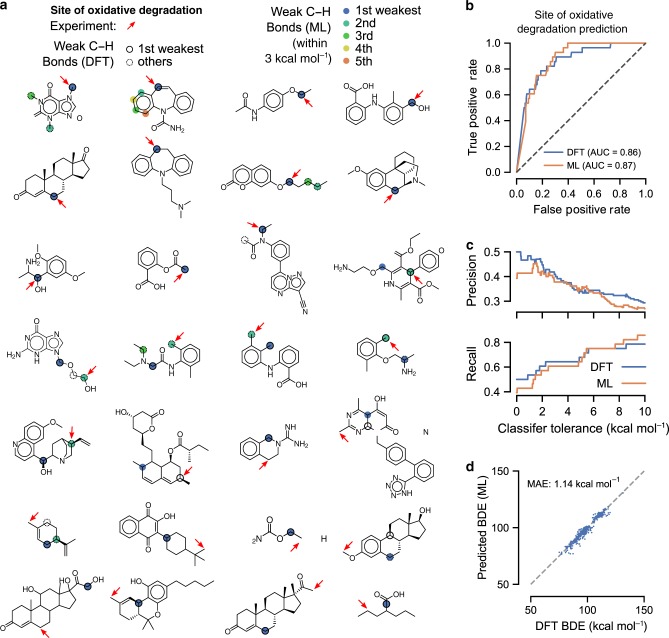
Fig. 7. Application of ALFABET to predict site of oxidative degradation.
a Structures of many of drug molecules where the site of oxidative degradation is known. Arrows indicate the experimentally determined breaking bond, while colors and circles indicate weakest bonds determined by ML and DFT, respectively. ML-predicted weakest bonds identify the experimental site in 11 out of the 28 molecules. b ROC curve for classifiers that predict the metabolic site through BDEs generated through ML or DFT. Both approaches yield similar performance. c Precision and recall of classifiers based on bond strengths calculated via DFT and ML approaches. Potential metabolic sites included all C–H bonds within a given energy from a molecule’s minimum. d Accuracy of the ML method in predicting BDEs for 82 large, drug-like molecules.