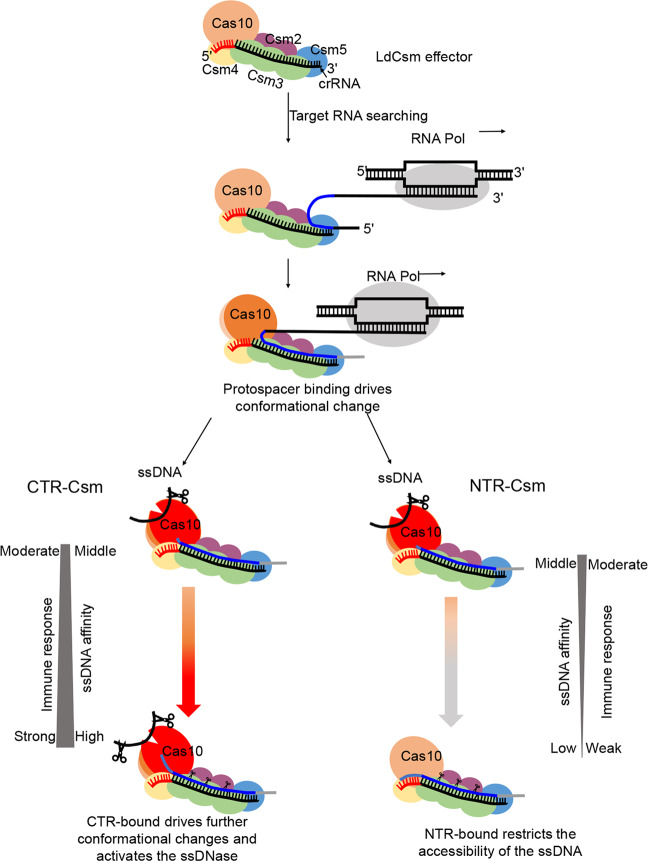
Fig. 7. Model of allosteric activation and repression of the LdCsm DNase.
The previous works have proposed the initial recognition of nascent transcript at the 5ʹ end of target RNA for type III complex, since both of Csm5 subunit in Csm complex and Cmr1 subunit in Cmr complex are crucial for target RNA binding36,37,68. These suggested that the binary LdCsm effector complex interacts with target transcript initially at the 5ʹ end of target RNA and further via sequence complementarity between the protospacer and the corresponding crRNA, leading to the formation of a ternary effector complex with a major conformational change. Addition of a single nucleotide at the 3ʹ-end of protospacer RNA results in an important allosteric change in the LdCsm DNase, giving an active enzyme. CTR-bound LdCsm exhibits the full level of substrate binding and DNA cleavage, whereas NTR-bound LdCsm closes the substrate-binding pocket, which deactivates the DNase. Finally, multiple Csm3 subunits cleave the target transcripts, and release of target RNA cleavage products restores the binary conformation, completing the spatiotemporal regulation of LdCsm systems.