Abstract
Purpose
Metal salts are used in formulation of dry powder inhalers (DPIs) for different purposes. Recently the role of these salts in production of small, dense but highly dispersible particles has emerged. In this study the effect of some such salts on dispersibility and respirability of spray dried levofloxacin formulations was evaluated in normal and reduced inhalation air flow or by increasing powder filling in capsules.
Methods
levofloxacin was co-spray dried with different concentrations of common metal chlorides (NaCl, KCl, CaCl2 and MgCl2) either with or without leucine as dispersibility enhancer. Particle size, moisture, morphology, triboelectrification tendency and fine particle fraction (FPF) of resulting powders were evaluated. In addition, the effect of these salts and leucine on dispersibility of resulting powders in reduced air flow rate and increased capsule filling mass were evaluated.
Results
Presence of higher tested concentrations of divalent cations increased water content, and reduced FPF significantly. Addition of leucine reduced water content and electrostatic charge, increased particle size and FPF and improved spray drying yield significantly. Lower concentrations of salts did not affect FPF of leucine containing powders significantly, but presence of 2.5% NaCl or MgCl2 preserved the dispersibility in higher capsule fillings. A 2.5% concentration of NaCl in such formulations preserved dispersibility in lower air flows.
Conclusion
Higher amounts of divalent salts increases triboelectrification and moisture absorption, and reduces FPF. Lower concentrations of NaCl could not improve FPF of leucine containing formulations significantly, but preserves dispersibility in low air flows and high capsule fillings.
Graphical abstract
Keywords: Dry powder inhaler, Spray drying, Levofloxacin, Metal salts, Leucine, Particle engineering
Introduction
Chronic pulmonary infection and inflammation, mainly infections caused by Pseudomonas aeruginosa (P. aeruginosa), may lead to respiratory failure and are known as the main cause of morbidity and mortality in cystic fibrosis (CF) patients [1]. Recently, the use of inhaled antibiotics for treatment of respiratory infections has received much attention. Local administration of antibiotics to lung allows direct delivery to the site of infection and therefore a lower dose is needed to achieve an adequate concentration [2, 3]. Studies have shown that the use of inhaled antibiotics reduces the frequency of pulmonary exacerbations and patient hospitalizations [4]. Therefore, inhaled antibiotics have become a common treatment against P. aeruginosa chronic infections [5].
Levofloxacin is a fluoroquinolone antibiotic, effective against P. aeruginosa. It was shown that levofloxacin retains its anti-pseudomonal activity in the sputum of CF patients in vitro whereas the activity of some other antibiotics such as tobramycin diminishes in such conditions [6]. Nebulized levofloxacin is well tolerated, has significant efficacy and may reduce the risk of resistance [7]. Using nebulizers is associated with some difficulties including complexity of the device, the need for preparation before and cleaning after each use and a relatively long time required for nebulization [8, 9]. On the other hand, dry powder inhalers (DPIs) allow fast delivery of high drug doses while the related device is readily portable and require simple cleaning [10]. Therefore, using a DPI formulation of levofloxacin can provide a better patient compliance by reducing costs and making administration easier while taking advantages of local delivery [11]. A dry powder formulation of Levofloxacin is under development by Pulmatrix Inc. (Lexington, Mass., USA) which according to the latest reports is in preclinical stage of development.
Employing relatively large sized particles (usually lactose) as carriers is a formulation strategy to enhance dispersibility of fine dry powders for inhalation. However, addition of carriers reduces the ratio of active ingredient that can be delivered. Hence, in case of antibiotics that higher doses are needed, carrier free formulations using particle engineering is taken into consideration. [3, 12, 13]
Salts of metal cations are used in formulations of inhalable dry powders as excipients for different purposes such as excipient enhanced growth [14] or stabilizing agents [15]. It has recently been reported that addition of small amounts of these metal cation salts and other excipients, results in formation of dense particles which are both geometrically and aerodynamically small in size. In contrast to conventional small particles that need carriers to avoid cohesion and maintain dispersibility, these particles are highly dispersible and have high emitted does even at low air flow rates. Because of high density and no need for lactose blending, these formulations can help deliver higher doses [16, 17]. There are few published reports on effect of these salts on dispersibility of formulations. It is reported in one study, that the presence of relatively small amounts of sodium chloride in dry powder formulation can result in small particles with good dispersibility in a range of flow rates, and is a simple and suitable pulmonary delivery system for high dose drugs [11]. In view of the above literature we decided to evaluate the effect of metal salts on aerosol performance of carrier free dry powder formulations for inhalation.
The aim of this study is to assess the effect of different metal salts on the aerosolization performance of spray dried carrier free powders of levofloxacin in presence or absence of leucine, as a well-known dispersing agent. A dry powder formulation of levofloxacin and leucine was proposed and optimized in our previous work [18]. In this work, magnesium and calcium as divalent and, potassium and sodium as monovalent metal cations were chosen in the form of chloride salts to challenge the effect of type and concentration of metal salts with each other and with leucine on physical characteristics and in vitro deposition of carrier free formulation of levofloxacin.
Materials and methods
Materials
Levofloxacin was kindly provided by Dr. Reddy, India. L-leucine, sodium chloride, potassium chloride, calcium chloride, magnesium chloride, n-octanol, dried methanol and hydrochloric acid 37% were purchased from Merck, Germany. Purified water was produced using a MilliQ gradient Plus Millipore systems. Size 3 hypromellose capsules were kindly provided by Cipla, India.
Spray drying
To prepare feed solution of each formulation, a total of 500 mg of ingredients by proportions described in Table 1 were dissolved in a quantity of water less than 20 mL. The solution pH was adjusted to a previously optimized value of 5.98 [18], by adding hydrochloric acid and using a Metrohm 827 pH meter (Metrohm Ltd., Belgium). Then the volume was adjusted to 20 mL to obtain a solution of 25 mg solutes per ml. In case of three-component formulations, leucine was added at a fix percentage of 21.79% (by levofloxacin weight) according to the results of our previous work [18]. Finally, solutions were spray dried using a Büchi Mini Spray Dryer B-191 (Büchi Labortechnik, Switzerland) in an open-loop mode. Spray dryer was equipped with a two-fluid flow atomizer nozzle of 0.7 mm diameter with a 10% feed rate setting equal to 4 mL per min. Air was used as drying gas and air flow rate was 600 L/h. Inlet temperature was adjusted at 150 °C and aspiration ratio was set to 90%. Three preparations of each formulation were produced and stored in 10 mL glass containers sealed with rubber stopper and aluminum ferrule fitting.
Table 1.
Compositions of the formulations and their physical characteristics
| Run | Leucine (%)* | Salt Type | Salt (%)* | Spray Drying Yield (%) | D50% (μm) | Water Content (%) | Electrostatic Charge (nColumb/mL) |
|---|---|---|---|---|---|---|---|
| 1 | 0 | MgCl2 | 2.5 | 29 ± 1.41 | 1.86 ± 0.63 | 7.56 ± 0.17 | −3.18 ± 0.27 |
| 2 | 0 | MgCl2 | 5 | 48 ± 1.53 | 2.25 ± 0.18 | 8.15 ± 0.07 | −3.35 ± 0.18 |
| 3 | 0 | MgCl2 | 10 | 53 ± 1.33 | 2.05 ± 0.45 | 11.23 ± 0.18 | −3.47 ± 0.39 |
| 4 | 0 | MgCl2 | 20 | 38 ± 1.72 | 1.74 ± 0.33 | 14.15 ± 0.12 | −4.65 ± 0.12 |
| 5 | 21.8 | MgCl2 | 2.5 | 43 ± 2.59 | 2.45 ± 0.23 | 5.29 ± 0.07 | −2.32 ± 0.13 |
| 6 | 21.8 | MgCl2 | 5 | 47 ± 3.32 | 2.41 ± 0.05 | 5.35 ± 0.05 | −2.50 ± 0.18 |
| 7 | 21.8 | MgCl2 | 10 | 52 ± 2.14 | 1.85 ± 0.07 | 6.31 ± 0.03 | −3.10 ± 0.22 |
| 8 | 21.8 | MgCl2 | 20 | 65 ± 3.27 | 1.80 ± 0.06 | 8.11 ± 0.06 | −4.12 ± 0.19 |
| 9 | 0 | CaCl2 | 2.5 | 28 ± 2.28 | 1.86 ± 0.38 | 8.03 ± 0.19 | −2.17 ± 0.32 |
| 10 | 0 | CaCl2 | 5 | 35 ± 1.87 | 2.67 ± 0.32 | 8.57 ± 0.12 | −2.28 ± 0.21 |
| 11 | 0 | CaCl2 | 10 | 33 ± 1.04 | 2.85 ± 0.49 | 10.22 ± 0.09 | −2.55 ± 0.12 |
| 12 | 0 | CaCl2 | 20 | 42 ± 1.29 | 2.49 ± 0.21 | 13.51 ± 0.16 | −3.03 ± 0.17 |
| 13 | 21.8 | CaCl2 | 2.5 | 42 ± 2.18 | 2.81 ± 0.12 | 6.09 ± 0.04 | −2.4 ± 0.11 |
| 14 | 21.8 | CaCl2 | 5 | 42 ± 1.39 | 2.89 ± 0.09 | 6.53 ± 0.09 | −2.25 ± 0.08 |
| 15 | 21.8 | CaCl2 | 10 | 58 ± 1.11 | 2.51 ± 0.08 | 8.68 ± 0.06 | −2.7 ± 0.15 |
| 16 | 21.8 | CaCl2 | 20 | 53 ± 1.42 | 2.32 ± 1.02 | 11.16 ± 0.05 | −3.34 ± 0.29 |
| 17 | 0 | KCl | 2.5 | 24 ± 3.1 | 1.45 ± 0.08 | 7.23 ± 0.05 | −2.05 ± 0.12 |
| 18 | 0 | KCl | 5 | 23 ± 2.9 | 1.74 ± 0.06 | 7.42 ± 0.03 | −2.11 ± 0.08 |
| 19 | 0 | KCl | 10 | 23 ± 2.5 | 1.55 ± 0.03 | 8.12 ± 0.07 | −2.52 ± 0.09 |
| 20 | 0 | KCl | 20 | 31 ± 3.6 | 1.53 ± 0.04 | 9.24 ± 0.04 | −2.38 ± 0.15 |
| 21 | 21.8 | KCl | 2.5 | 54 ± 1.9 | 2.30 ± 0.03 | 6.15 ± 0.04 | −2.19 ± 0.04 |
| 22 | 21.8 | KCl | 5 | 51 ± 2.6 | 1.81 ± 0.05 | 6.11 ± 0.05 | −2.15 ± 0.07 |
| 23 | 21.8 | KCl | 10 | 50 ± 3.4 | 1.80 ± 0.04 | 6.03 ± 0.08 | −2.3 ± 0.03 |
| 24 | 21.8 | KCl | 20 | 48 ± 2.8 | 1.50 ± 0.05 | 6.32 ± 0.07 | −3.1 ± 0.09 |
| 25 | 0 | NaCl | 2.5 | 26 ± 2.3 | 1.65 ± 0.17 | 6.17 ± 0.09 | −2.18 ± 0.07 |
| 26 | 0 | NaCl | 5 | 26 ± 1.9 | 2.09 ± 0.18 | 6.14 ± 0.07 | −2.35 ± 0.09 |
| 27 | 0 | NaCl | 10 | 35 ± 2.1 | 1.83 ± 0.13 | 6.52 ± 0.04 | −2.39 ± 0.09 |
| 28 | 0 | NaCl | 20 | 34 ± 3.6 | 1.59 ± 0.19 | 7.2 ± 0.11 | −2.8 ± 0.06 |
| 29 | 21.8 | NaCl | 2.5 | 54 ± 2.0 | 2.44 ± 0.11 | 5.0 ± 0.13 | −1.92 ± 0.10 |
| 30 | 21.8 | NaCl | 5 | 50 ± 1.8 | 2.48 ± 0.12 | 4.9 ± 0.03 | −2.18 ± 0.09 |
| 31 | 21.8 | NaCl | 10 | 55 ± 1.8 | 2.34 ± 0.09 | 5.2 ± 0.07 | −2.24 ± 0.08 |
| 32 | 21.8 | NaCl | 20 | 61 ± 1.1 | 2.16 ± 0.04 | 5.2 ± 0.06 | −2.89 ± 0.12 |
| 33 | 21.8 | – | – | 51.9 ± 1.15 | 2.45 ± 0.08 | 5.19 ± 0.08 | −1.55 ± 0.19 |
| 34 | 0 | – | – | 25.4 ± 1.70 | 1.31 ± 0.11 | 6.29 ± 0.09 | −4.15 ± 0.32 |
*All percentages are relative to Levofloxacin concentration
Powder characterization
The following properties were investigated for each of three preparation of every formulation.
Spray drying yield
Yield was calculated as the ratio of the weight of powder collected from collection chamber, to the total weight of solids in the feed solution, which was 500 mg as mentioned above.
Water content
The amount of water in each formulation was measured by Karl Fischer (KF) titration method using a K758 (Metrohm Ltd., Belgium). Water content was determined by automatic titration against Karl Fischer reagent A (Merck, Germany), after dissolving the powder in dried methanol (containing less than 0.003% water) (Merck, Germany).
Particle size distribution
Laser light diffraction was employed for analyzing particle size distribution of spray dried powders. An approximate amount of about 10 mg from each powder was dispersed in 10 mL n-octanol as dispersing media and sonicated in an ultrasonic bath (Liarre, Italy) for 30 s. Particle size of suspended powder was measured immediately after sonication, using a Mastersizer X (Malvern, UK). The suspension was gradually added to measuring cell, containing n-octanol, to reach an obscuration around 20%. The process was repeated three times for every prepared powder and the median diameter (D50%) was recorded.
Scanning Electron microscopy and energy dispersive X-ray spectroscopy
Scanning electron micrographs of selected formulations were obtained using a HITACHI S-4160 field emission scanning electron microscope (Amsterdam, Netherland) with an acceleration voltage of 30 kV and 10.0 K magnification. Samples were prepared by scattering a small amount of powder on a carbon sticky tape to form a sparse layer. Then the tape was placed on stubs and gold deposited under vacuum condition. Images were taken to assess and compare the particle morphology.
Energy Dispersive X-ray Spectroscopy (EDX) was performed on some selected powders using a MIRA 3-XMU field emission scanning electron microscope (TESCAN, Czech Republic) equipped with energy dispersive X-ray Spectroscopy (EDX). The elemental analysis by EDX was done on two sets of selected windows, once on the edge of the particles to evaluate the surface composition, and another time around the center of a particles that reflects the elemental composition of internal parts of particles as well. For each sample three windows on particle edge and three around center were selected and the average elemental composition results for these windows were calculated. Similar method was employed for analyzing the particle composition in previous studies [19, 20].
X-ray diffraction (XRD)
Crystallinity of powders was studied using a D5000 X-ray Diffractometer (Siemens, Germany), while 40 kV Cu-Ka radiation and 30 mA current were used. Illumination area was kept constant (10*20 mm2) by variable slits. Scanning was done in 0.090° steps (step time, 1.0 s) from 5 to 75° in 2 theta scale.
Electrostatic charge of powders
Triboelectrification tendency of powders was evaluated during spray drying process using the setup explained in a previous report by the authors [18]. Briefly, electrostatic charge from particles is transferred, by collision or induction, to a stainless-steel net located between the drying chamber and cyclone of spray dryer set. This conducting net is connected to one plate of a capacitor with defined capacity. The other plate of capacitor is earthed. The electric potential difference in millivolts (mV) generated between capacitor plates during spray drying of powders was plotted against time in seconds (s). The rate of change in electric potential is used to calculate the rate of change in charge in nanocoulombs per seconds (nC/s) for each formulation using Q = C.V equation, in which Q is charge in nanocoulombs, C is capacitance in nano-farads, and V is voltage in millivolts. Measurement was done in triplicate and the final result was stated as average of three charge evaluation results. Because the flow rate and solid concentration of feed solution during spray drying of all formulations were set on a definite value, the amount of change in charge in time is indicative of charge in a definite amount of powder and could be used as a factor for comparing triboelectrification tendency of the powders.
Aerodynamic characterization
Fine particle fraction of spray dried powders was assessed using an Anderson cascade impactor (Copley, Nottingham, UK) equipped with a pre-separator (United States Pharmacopoeia (USP) apparatus 3). A Copley vacuum pump (Copley, Nottingham, UK) was employed to run an air flow rate of 60 L/min. This flow rate was adjusted using a digital flow meter (ERW-DFM, Erweka, Germany). Impactor was assembled according to USP 40. Size 3 hypromellose capsules were filled with 20 mg of each dry powder preparation and inserted in an Aerolizer® (Novartis, Switzerland) dry powder inhaler device. This device was fitted by the aid of a rubber fitting to the cascade impactor’s entrance and the flow was run for 4 s allowing 4 L of air pass through the device.
Finally, the impactor was disassembled and different stages rinsed with purified water separately and the amount of drug on each stage were analyzed using a UV-spectrophotometer (SPEKOL 2000, Analytik Jena AG, Germany) at 290 nm, according to a previously reported method [21].
The sum of the amounts of levofloxacin collected from all stages, including inhaler device and capsule was named the Recovered Dose (RD). A recovered dose above 90% of total capsule loaded dose was considered necessary for accepting the results of this analysis. Fine Particle Dose (FPD) was defined as the amount of powder deposited on all stages from stage 2 to the filter. Cut-off diameter of stage two in 60 L/min flow rate is about 3.2 μm according to USP 40 and other reports [22]. Fine Particle Fraction (FPF) was calculated as the percentage of FPD to RD.
Effect of capsule filling on aerosolization performance
The effect of powder mass loaded in capsules, on aerosolization performance of formulations was assessed. Capsules were filled with either 20 or 40 mg of spray dried powders of selected formulations and the FPF analysis was done as described before. The amount of powders emitted from the device, or percent emitted dose (ED) was calculated as the percentage of drug mass collected from all stages of cascade impactor, except the device, to RD. Resulting FPF and ED values from capsules filled with 40 mg of a formulation was compared to the values resulted from capsules filled with 20 mg of that formulation. The process was repeated three times for every formulation and the average difference, in percent, of FPF and ED values resulted from two different fillings was calculated for every formulation. Changes in FPF and ED resulting from increasing capsule filling for different formulations were compared to evaluate the effect of capsule filling on dispersibility and respirability of different formulation.
Effect of air flow rate on aerosolization performance
Effect of different inspiration air flow rate on the FPF and ED of selected formulations was also studied. Analysis was conducted with the same process described above, at two different air flow rates, 28.3 or 60 L/min. Values of FPF and ED were measured for each formulation at both flow rates in triplicate and the average percentage of change in these parameters by changing flow rate were calculated for every formulation. Percentage of change in FPF and ED by decreasing flow rate was compared among different formulations to evaluate the effect of different flow rates on aerosolization of those formulations.
Statistical analysis
All data derived from experiments are reported as the mean ± standard deviation (SD) of triplicate analysis of each formulation. One-way analysis of variance with Tukey’s post-hoc test, and p < 0.05 was employed as statistical test to compare among groups of results wherever the values are claimed to be statistically compared.
Results and discussions
Physicochemical characteristics
Spay drying yield
Yield of spray drying is a measure of the productivity of this process [23]. When levofloxacin was spray dried alone, yield was 25.4 ± 1.70% (Table 1) and most of the powder deposited on the cyclone wall, which can be due to higher adhesive and cohesive forces resulted from smaller particle size and higher electrostatic charge. Addition of higher proportions of metal salts to drug formulation, significantly increased the process yield, probably due to the change in particles morphology and electrostatic charge which will be discussed later. This change in yield was dependent on type and percentage of the salt used in formulation. On the other hand, leucine also improved the productivity of the process significantly. It is well known that leucine accumulates on the surface of particles during spray drying process and improves the flowability of particles and increases the yield of process. The effect of leucine was in agreement with our previous report [18]. The results of this experiment showed that leucine can preserve its effect on the process yield in presence of different amounts of metal salts in formulations.
Water content
The residual water in spray dried formulations may affect both physical characteristics and the chemical stability of powders [23]. When either KCl, CaCl2 or MgCl2 is present in initial solutions containing levofloxacin, water content of the spray dried formulations was significantly increased by increasing salt concentration (Table 1). This rise was more significant for formulations containing divalent cation salts comparing to formulation of the drug alone. These salts are highly hygroscopic and therefore tend to preserve more water molecules during drying phase of the spray drying process, and on the other hand may more readily absorb atmospheric humidity subsequent to drying [24]. Hygroscopic powders have higher risk of hygroscopic growth and instability induced by moisture uptake and local recrystallization leading to particle aggregation which may adversely affect particle dispersion and deposition in lungs [25]. In contrast to other salts, no significant change was observed in water content of formulations containing NaCl as salt concentration is increased up to 10% w/w.
Incorporation of leucine in spray dried formulations resulted in a significant decrease in water content of all formulations. Addition of leucine to formulations containing divalent cation salts, exerted a more significant decreasing impact on water content of resulting formulations. Reduction in water content as a result of addition of hydrophobic amino acids such as leucine was reported in previous literature [26]. Furthermore, it was shown that sufficient amounts of leucine in formulation, exerts moisture protective properties as a result of its enrichment on particles surface and formation of a hydrophobic crust around them, which in turn prevents water sorption after formation of particles [27, 28].
Particle size
Particle size of spray dried formulations was measured and are reported in Table 1. All formulations had D50% between 1.31–2.89 μm, which was suitable for deep lung deposition [3, 15]. Spray dried levofloxacin formulation produced in the absence of other ingredients, presented the lowest D50% among all processed formulations. Accumulation of leucine on the surface of drying droplets as well as its hydrophobic nature provides a suitable medium for its crystallization which ends in formation of corrugated or collapsed outer shell and hence larger particles [29].
SEM images and EDX analysis
Scanning electron micrographs of different samples are presented in Fig. 1. Co-spray drying of the drug with divalent cation salts led to a substantial change in surface morphology of produced particles. When levofloxacin was spray dried alone, particles with rough and angular surfaces were produced (Fig. 1a). But as divalent cation salts were added to the primary solution, particle surfaces became smooth but dimpled (Fig. 1b and d). In contrast, co-processing of levofloxacin in presence of monovalent cation salts resulted in formation of particles with rough and angular surfaces (Fig. 1f and h). This is more obvious in formulations containing NaCl.
Fig. 1.
Scanning Electron Micrograms of Different Powders. A: SD Levo; B: SD Levo +2.5% MgCl2; C: SD Levo + Leu + 2.5% MgCl2; D: SD Levo +2.5% CaCl2; E: SD Levo + Leu + 2.5% CaCl2; F: SD Levo +2.5% KCl; G: SD Levo + Leu + 2.5% KCl; H: SD Levo +2.5% NaCl; I: SD Levo + Leu + 2.5% NaCl. *Levo: Levofloxacin, **SD: Spray Dried, ***Leu: Leucine
When leucine was added to formulations containing the drug and each of the cations, regardless of the type of cation, similar corrugated particles were produced (Fig. 1c, e, g and, i). This is a common structure for particles containing sufficient amounts of leucine on surface [30]. This structure reduces contact points among particles and reduces adhesive and cohesive forces comparing to particles with smooth surface. Therefore, such particles would have better dispersibility and flowability which results in better spray drying yield [31].
Results of EDX analysis (Table 2) proved this surface enrichment. Mass fraction of Fluorine element, which is only present in levofloxacin molecules, and mass fraction of each metal element on the surface of spray dried particles of leucine containing formulations were measured. The resulting data sets were compared to expected weight fraction of these elements on the surface of particles in a proportionate distribution of ingredients throughout the spray dried particles. Results showed a significant difference between measured surface concentration of these components and the expected concentrations. As both levofloxacin and cations had less fractions on the surface of particles than expected, it can be concluded that leucine was accumulated on the surface of particles. Therefore, because particle surface in these formulations is mostly consisted of leucine, the surface morphology of the particles was mainly governed by leucine, and particles became corrugated.
Table 2.
Percentage material compositions of spray dried formulations calculated from EDX analysis
| Theoretical surface composition | Measured surface composition | |||||
|---|---|---|---|---|---|---|
| Formulations | % Levofloxacin | % Leucine | % Salt | % Levofloxacin | % Leucine | % Salt |
| Levo + Leu + 2.5% MgCl2 | 80.45 | 17.54 | 2.01 | 45.27 ± 6.82 | 54.08 ± 6.82 | 0.65 ± 0.23 |
| Levo + Leu + 2.5% CaCl2 | 80.45 | 17.54 | 2.01 | 38.99 ± 5.32 | 60.34 ± 5.32 | 0.66 ± 0.05 |
| Levo + Leu + 2.5% KCl | 80.45 | 17.54 | 2.01 | 54.96 ± 7.13 | 44.21 ± 7.13 | 0.83 ± 0.41 |
| Levo + Leu + 2.5% NaCl | 80.45 | 17.54 | 2.01 | 27.96 ± 3.60 | 70.67 ± 3.62 | 1.37 ± 0.25 |
X-ray diffraction
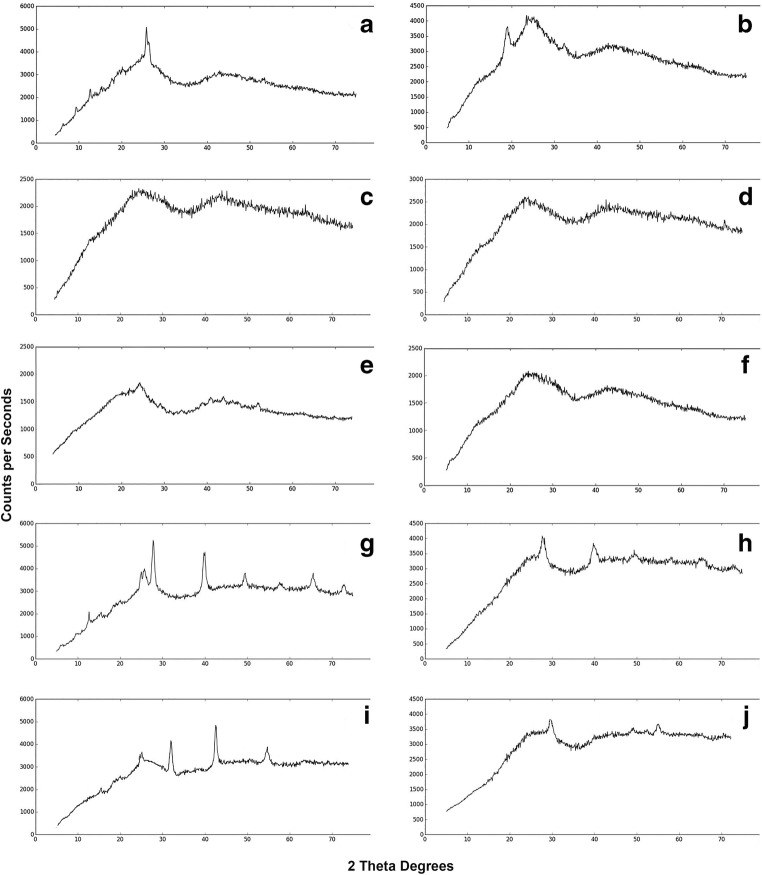
X-ray diffractograms of spray dried powders are presented in Fig. 2. Diffractograms of formulations containing divalent cation salts represented a mostly amorphous structure (Fig. 2c-f). For all other formulations, especially formulations containing only monovalent cations salts, diffractograms reflect a prominently amorphous state with some small peaks, which represents a partially crystalline structure. Presence of this structure may explain the rough and angular morphology observed on the surface of particles in SEM images of formulations containing monovalent cation salts and levofloxacin. When leucine was also added to these formulations, intensity of the peaks was reduced and the structure became more amorphous (compare Fig. 2g and i with Fig. 2h and j, respectively).
Fig. 2.
X-Ray Diffractograms of Spray Dried Formulations. a: SD Levo; b: SD Levo + Leu; c: SD Levo +2.5% MgCl2; d: SD Levo + Leu + 2.5% MgCl2; e: SD Levo +2.5% CaCl2; f: SD Levo + Leu + 2.5% CaCl2; g: SD Levo +2.5% KCl; h: SD Levo + Leu + 2.5% KCl; i: SD Levo +2.5% NaCl; j: SD Levo + Leu + 2.5% NaCl. *Levo: Levofloxacin, **SD: Spray Dried, ***Leu: Leucine
Electrostatic charge
Electrostatic charge of spray dried particles was measured online during the process and the results are included in Table 1. Generally, presence of metal salts decreased the electrostatic charge of samples as compared to −3.99 ± 0.46 value which was detected in our previous work for levofloxacin powder spray dried alone at the same pH [18]. This effect, beside the change in particle surface morphology, might be a reason for higher process yield that was observed upon adding metal salts to the formulations. By increasing the metal cation concentration in levofloxacin formulations in the absence of leucine, electrostatic charge was gradually increased. This increase was more significant with divalent cation salts. When leucine was also present in formulation, probably because of formation of outer leucine shell which was discussed before, particles generally showed lower tendency for triboelectrification [18]. Only in the presence of higher concentrations of divalent cation salts, a significant increase in charge was observed. Higher electrostatic charge or triboelectrification tendency of these powders may cause higher adhesive and cohesive forces and impair powder dispersibility [32, 33].
Aerodynamic characterization
The effect of metal salts on aerodynamic behavior of levofloxacin containing formulations was also dependent on the type of cations used in formulations. Formulations produced by co-spray drying of the drug with MgCl2 had lower FPF compared to formulations containing other cations (Fig. 3). This difference might be due to the higher electrostatic charge of MgCl2 containing particles (Table 1). For formulations containing 2.5 to 10% CaCl2, KCl or NaCl, FPF values were approximately identical. Again, different behaviors were observed among spray dried powders containing 20% of different metal salts. For samples containing 20% CaCl2, FPF was significantly decreased comparing with samples containing lower ratios of this salt. On the contrary, for formulations co-spray dried with 20% KCl or NaCl FPF was higher comparing to formulations containing lower amounts of these monovalent cations. Water content as a function of hygroscopicity of divalent cation salts may have caused this trend of FPF. High moisture content observed in samples containing 20% divalent cation salts may lead to higher adhesive and cohesive forces for these particles. Thin layer of water on particle surface gives rise to capillary forces which induces attractive forces between contiguous particle surfaces [34].
Fig. 3.
Effect of different concentrations of cations (a: Mg2+, b: Ca2+, c: K+ and d: Na+) in the presence or absence of Leu on FPF% of the resulting powders
For all leucine containing formulations, FPF was significantly higher than formulations prepared without leucine (Fig. 3). The trend of FPF changes with increasing salt concentration in leucine containing formulations, was again dependent on the type of cations. The most profound change in the FPF, by addition of leucine, was observed in formulations containing MgCl2. For formulations containing monovalent cations and leucine, FPF did not change significantly by increasing salt concentration. In contrast, for formulations containing salts of divalent cations, FPF was significantly reduced by increasing salt ratio. This trend can be explained by the effect of hygroscopicity of salts and higher water content of these powders. The protective effect of amino acids on water absorption was reported previously for disodium cromoglycate as a model hygroscopic drug [27]. But according to data presented in Table 1, leucine could not effectively protect against moisture sorption in particles containing highly hygroscopic CaCl2 as metal salt. Thus, tri-component formulations containing 20% of this salt showed the lowest FPF among all other tri-component formulations produced in this work. In these series of formulations, the highest FPF was observed for powders composed of levofloxacin, leucine and 2.5% NaCl or MgCl2. Briefly, by presenting two formulations, Lawlor et al. reported that addition of a fixed proportion of magnesium or sodium cations on formulation of levofloxacin led to production of small, dense and respirable particles which preserved levofloxacin content and purity upon storage in different conditions [35]. But they mentioned nothing about the amount of these salts and the effects of various percentages of them on physical properties and aerosolization characteristics of formulations.
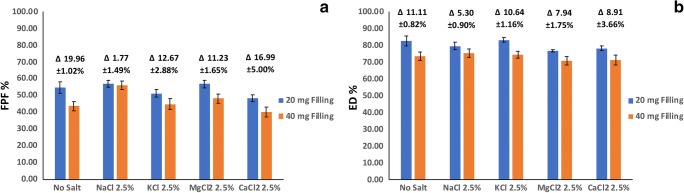
Effect of capsule filling on aerosolization performance
To assess the effect of metal salts on aerosolization performance of powders from capsules of different filling masses, formulations of levofloxacin containing 21.8% leucine and 2.5% of different metal salts were prepared. Minimum concentration of salts was chosen because of the complex and various effect of higher concentration of different salts described above. A formulation containing levofloxacin and leucine without cations was also tested as control sample.
As the amount of powder filled in capsules increased from 20 mg to 40 mg, the FPF and ED values measured for control formulation were decreased by 19.96 ± 1.02% and 11.11 ± 0.82%, respectively (Fig. 4). The effect of metal salts on aerosolization behavior of powders from different capsule filling masses was variable by cation type. Spray dried powders containing NaCl as metal salt, exhibited the least reduction in the FPF (1.77 ± 1.49%) and ED (5.30 ± 0.90%) by increasing the filling dose. MgCl2 containing powders took the second place in exhibiting least reduction in FPF and ED, as a result of increasing capsule filling mass. For formulations co-spray dried with other salts (KCl and CaCl2) the percentage of change in FPF or ED by increasing capsule filling was statistically similar to that of control group. These results indicate that the presence of small amounts of NaCl in DPI formulation may allow using higher loading mass in the capsules with the least negative effects on dispersibility and respirability of aerosolized drug.
Fig. 4.
Effect of capsule filling mass on (a) FPF and (b) ED of levofloxacin aerosolized from different formulations containing the drug, leucine with or without 2.5% of salts. Δ values express the difference between FPF and ED amounts in two different capsule fillings
Effect of air flow rate on aerosolization performance
Amounts of FPF and ED for spray dried formulations containing levofloxacin, leucine and 2.5% salts were also measured at different air flow rates (28.3 L/min and 60 L/min). For control powder, comprising levofloxacin and leucine without metal salts, 43.85 ± 3.82% drop in FPF and 43.57 ± 1.16% drop in ED was observed when air flow rate was reduced from 60 L/min to 28.3 L/min (Fig. 5). All formulations containing metal salts showed significantly smaller decrease in FPF and ED by decreasing inspiration air flow rate, comparing with control formulation. As an exception to this, for potassium containing formulations, percentage of change in ED was not markedly different from that of control formulation.
Fig. 5.
Effect of air flow rate (L/min) on (a) FPF and (b) ED of levofloxacin aerosolized from different formulations containing the drug, leucine with or without 2.5% of salts. Δ values express the difference between FPF and ED amounts in two different air flows
Again, with powders containing NaCl, the difference between FPF and ED upon aerosolization of the powder in two air flow rates were significantly less than the measured difference for other formulations. Therefore, the addition of 2.5% NaCl to levofloxacin and leucine formulation, could preserve their dispersibility and respirability in lower air flow rates. This is in agreement with previous report which presented the effect of sodium chloride on dispersibility of a levofloxacin dry powder formulation over a range of air flow rates [11].
Conclusion
This study investigated the effect of monovalent and divalent cation salts on aerosolization performance of levofloxacin dry powder formulations spray dried with or without leucine. Only higher concentrations of monovalent cation salts added to levofloxacin, could significantly increase the FPF of resulting powder. Presence of divalent cations salts diminished the FPF of particles especially at higher concentrations due both to higher electrostatic charge and higher water content of resulting powder. Presence of leucine as the third component in spray dried formulations caused a profound increase in aerosol performance of drug containing powders. Leucine by surface enrichment reduced electrostatic charge and exhibited a protective effect against moisture absorption by powders. It was shown that, among studied salts, presence of NaCl in formulations helped preserve dispersibility and respirability of powders in different filling masses and under different air flow rates.
Funding information
This research has been supported by Tehran University of Medical Sciences & health Services [grant number 94–01–33-28,742].
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stass H, Weimann B, Nagelschmitz J, Rolinck-Werninghaus C, Staab D. Tolerability and pharmacokinetic properties of ciprofloxacin dry powder for inhalation in patients with cystic fibrosis: a phase I, randomized. Dose-Escalation Study Clin Ther. 2013;35:1571–1581. doi: 10.1016/j.clinthera.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Dudley MN, Loutit J, Griffith DC. Aerosol antibiotics: considerations in pharmacological and clinical evaluation. Curr Opin Biotechnol. 2008;19:637–643. doi: 10.1016/j.copbio.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Zhou QT, Leung SSY, Tang P, Parumasivam T, Loh ZH, Chan HK. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv Drug Deliv Rev. 2015;85:83–99. doi: 10.1016/j.addr.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Flume PA, O’Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ, Willey-Courand DB, et al. Cystic fibrosis pulmonary guidelines. Am J Respir Crit Care Med. 2007;176:957–969. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 5.Tiddens HAWM, Bos AC, Mouton JW, Devadason S, Janssens HM. Inhaled antibiotics: dry or wet? Eur Respir J. 2014;44:1308–1318. doi: 10.1183/09031936.00090314. [DOI] [PubMed] [Google Scholar]
- 6.Gibson RL, Retsch-Bogart GZ, Oermann C, Milla C, Pilewski J, Daines C, Ahrens R, Leon K, Cohen M, McNamara S, Callahan TL, Markus R, Burns JL. Microbiology, safety, and pharmacokinetics of aztreonam lysinate for inhalation in patients with cystic fibrosis. Pediatr Pulmonol. 2006;41:656–665. doi: 10.1002/ppul.20429. [DOI] [PubMed] [Google Scholar]
- 7.Geller DE, Flume PA, Griffith DC, Morgan E, White D, Loutit JS, et al. Pharmacokinetics and safety of MP-376 (levofloxacin inhalation solution) in cystic fibrosis subjects. Antimicrob Agents Chemother. 2011;55:2636–2640. doi: 10.1128/AAC.01744-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.d’Angelo I, Conte C, La Rotonda MI, Miro A, Quaglia F, Ungaro F. Improving the efficacy of inhaled drugs in cystic fibrosis: challenges and emerging drug delivery strategies. Adv Drug Deliv Rev. 2014;75:92–111. doi: 10.1016/j.addr.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Ting L, Aksenov S, Bhansali SG, Ramakrishna R, Tang P, Geller DE. Population pharmacokinetics of inhaled tobramycin powder in cystic fibrosis patients. CPT Pharmacometrics Syst Pharmacol. 2014;3:e99. doi: 10.1038/psp.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kesser KC, Geller DE. New aerosol delivery devices for cystic fibrosis. Respir Care. 2009;54:754–768. doi: 10.4187/002013209790983250. [DOI] [PubMed] [Google Scholar]
- 11.Manzanedo D, Brande M, Kramer SR, Yee LW, Dehaan WH, Clarke RW, et al. Formulation characterization of a novel levofloxacin pulmonary dry powder drug delivery technology. Respir. Drug Deliv. 2012;2012:713–716. [Google Scholar]
- 12.Weers JG, Bell J, Chan H-K, Cipolla D, Dunbar C, Hickey AJ, et al. Pulmonary Formulations: What Remains to be Done? J Aerosol Med Pulm Drug Deliv. 2010;23:S-5–S-23. doi: 10.1089/jamp.2010.0838. [DOI] [PubMed] [Google Scholar]
- 13.Pilcer G, De Bueger V, Traina K, Traore H, Sebti T, Vanderbist F, et al. Carrier-free combination for dry powder inhalation of antibiotics in the treatment of lung infections in cystic fibrosis. Int J Pharm. 2013;451:112–120. doi: 10.1016/j.ijpharm.2013.04.069. [DOI] [PubMed] [Google Scholar]
- 14.Tian G, Longest PW, Li X, Hindle M. Targeting aerosol deposition to and within the lung airways using excipient enhanced growth. J Aerosol Med Pulm Drug Deliv. 2013;26:248–265. doi: 10.1089/jamp.2012.0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Healy AM, Amaro MI, Paluch KJ, Tajber L. Dry powders for oral inhalation free of lactose carrier particles. Adv Drug Deliv Rev. 2014;75:32–52. doi: 10.1016/j.addr.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Srinivasan G, Shetty A. Advancements in dry powder inhaler. Asian J Pharm Clin Res. 2017;10:8. doi: 10.22159/ajpcr.2017.v10i2.14282. [DOI] [Google Scholar]
- 17.Lipp MM, Sung JC. Cationic dry powders. US Patent 9,744,130, 2017.
- 18.Barazesh A, Gilani K, Rouini M, Barghi MA. Effect of pH and leucine concentration on aerosolization properties of carrier-free formulations of levofloxacin. Eur J Pharm Sci. 2018;118:13–23. doi: 10.1016/j.ejps.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Kaialy W, Hussain T, Alhalaweh A, Nokhodchi A. Towards a more desirable dry powder inhaler formulation: large spray-dried mannitol microspheres outperform small microspheres. Pharm Res. 2014;31:60–76. doi: 10.1007/s11095-013-1132-2. [DOI] [PubMed] [Google Scholar]
- 20.Kho K, Hadinoto K. Effects of excipient formulation on the morphology and aqueous re-dispersibility of dry-powder silica nano-aggregates. Colloids Surfaces A Physicochem Eng Asp. 2010;359:71–81. doi: 10.1016/j.colsurfa.2010.01.066. [DOI] [Google Scholar]
- 21.Maleque M, Hasan MR, Hossen F, Safi S. Development and validation of a simple UV spectrophotometric method for the determination of levofloxacin both in bulk and marketed dosage formulations. J Pharm Anal. 2012;2:454–457. doi: 10.1016/j.jpha.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols SC, Brown DR, Smurthwaite M. New Concept for the Variable Flow Rate Andersen Cascade Impactor and Calibration Data. J Aerosol Med. 1998;11. [PubMed]
- 23.Belotti S, Rossi A, Colombo P, Bettini R, Rekkas D, Politis S, Colombo G, Balducci AG, Buttini F. Spray dried amikacin powder for inhalation in cystic fibrosis patients: a quality by design approach for product construction. Int J Pharm. 2014;471:507–515. doi: 10.1016/j.ijpharm.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 24.Albayrak C, Barım G, Dag Ö. Effect of hygroscopicity of the metal salt on the formation and air stability of lyotropic liquid crystalline mesophases in hydrated salt–surfactant systems. J Colloid Interface Sci. 2014;433:26–33. doi: 10.1016/j.jcis.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Mickey AJ, Gonda I, Irwin WJ, FJT F. Effect of hydrophobic coating on the behavior of a hygroscopic aerosol powder in an environment of controlled temperature and relative humidity. J Pharm Sci. 1990. [DOI] [PubMed]
- 26.Yang X-F, Xu Y, Qu D-S, Li H-Y. The influence of amino acids on aztreonam spray-dried powders for inhalation. Asian J Pharm Sci. 2015;10:541–548. doi: 10.1016/j.ajps.2015.08.002. [DOI] [Google Scholar]
- 27.Li L, Sun S, Parumasivam T, Denman JA, Gengenbach T, Tang P, Mao S, Chan HK. L-Leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders. Eur J Pharm Biopharm. 2016;102:132–141. doi: 10.1016/j.ejpb.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Vehring R. Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25:999–1022. doi: 10.1007/s11095-007-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangal S, Meiser F, Tan G, Gengenbach T, Denman J, Rowles MR, Larson I, Morton DA. Relationship between surface concentration of l-leucine and bulk powder properties in spray dried formulations. Eur J Pharm Biopharm. 2015;94:160–169. doi: 10.1016/j.ejpb.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 30.Feng AL, Boraey MA, Gwin MA, Finlay PR, Kuehl PJ, Vehring R. Mechanistic models facilitate efficient development of leucine containing microparticles for pulmonary drug delivery. Int J Pharm. 2011;409:156–163. doi: 10.1016/j.ijpharm.2011.02.049. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Okuda T, Lu X-Y, Chan H-K. Amorphous powders for inhalation drug delivery. Adv Drug Deliv Rev. 2016;100:102–115. doi: 10.1016/j.addr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Karner S, Anne UN. The impact of electrostatic charge in pharmaceutical powders with specific focus on inhalation-powders. J Aerosol Sci. 2011;42:428–445. doi: 10.1016/j.jaerosci.2011.02.010. [DOI] [Google Scholar]
- 33.Murtomaa M, Savolainen M, Christiansen L, Rantanen J, Laine E, Yliruusi J. Static electrification of powders during spray drying. J Electrost. 2004;62:63–72. doi: 10.1016/j.elstat.2004.05.001. [DOI] [Google Scholar]
- 34.Price R, Young PM, Edge S, Staniforth JN. The influence of relative humidity on particulate interactions in carrier-based dry powder inhaler formulations. Int J Pharm. 2002. [DOI] [PubMed]
- 35.Lawlor CP, Tauber MK, Brogan JT, Zhu L, Currie DF, Trautman BG, et al. Levofloxacin dry powders engineered for efficient pulmonary delivery and stability. Respir Drug Deliv. 2014;2014:549–552. [Google Scholar]