Abstract
Purpose
The aim of this study was to create and assess biological activity of a new compound based on carnosine and acetylsalicylic acid (ASA) that will comprise antioxidant effect with antiplatelet activity, while simultaneously preventing side effects on the gastrointestinal tract.
Methods
Salicyl-carnosine (SC) was synthesized by condensation of ASA and carnosine. Antioxidant activity was determined by spectrophotometric and chemiluminescence methods. Antiplatelet activity was carried out by the light transmission-aggregometry method using the inductor ADP. Chronic gastric ulcer in rats was modeled using glacial acetic acid.
Results
Using SOD-like activity, iron-induced chemiluminescence, BaSO4-activated respiratory burst, and evaluation of red blood cell structure stabilization during oxidative damage induced by sodium hypochlorite, it was shown that SC possesses antioxidant activity analogous, or better, than that of carnosine. Antiplatelet activity of SC was evaluated in the blood of healthy individuals, and was also shown to be comparable to, or exceeding that of ASA. Also SC demonstrates high resistance to hydrolysis by tissue and serum carnosinases. Most importantly, it was shown that SC has protected the gastric mucosa against the formation of stomach ulcerative lesions and promoted their epithelization, therefore overcoming the undesirable inherent side effects of ASA.
Conclusions
SC preserves pharmacologically significant properties of ASA and carnosine while retaining an anti-ulcer activity and resistance to the carnosinase hydrolysis at the same time. These properties are particularly promising for the potential development of new anti-inflammatory and antithrombotic drugs.
Graphical abstract.
.
Electronic supplementary material
The online version of this article (10.1007/s40199-019-00323-x) contains supplementary material, which is available to authorized users.
Keywords: Carnosine, Acetylsalicylic acid, Salicylic acid, Antioxidant, Antiplatelet action, Gastrointestinal ulcer
Introduction
Salicylic acid (SA) and its derivatives have long been used in medicine as non-steroidal anti-inflammatory drugs (NSAIDs). The most well-known salicylate is acetylsalicylic acid (ASA, aspirin) [1, 2]. In addition to being used for its anti-inflammatory, antipyretic, and analgesic effects ASA is used in the prevention and treatment of cardiovascular diseases [2–6] due to its high ability to inhibit platelet aggregation. The antithrombotic effect of ASA is accounted to its acetyl group, which irreversibly inhibits cyclooxygenase 1 (COX-1) by means of acetylation and, as a consequence, suppresses thromboxane A2 formation [3].
The bioavailability of ASA is approximately 40–50% after oral administration in therapeutic doses. During this process ASA is converted to SA by deacetylation over the course of 15–20 min. The pharmacological action of the latter includes anti-inflammatory, antipyretic and analgesic effects [7–9].
The antioxidant activity, which is inherent to salicylates, is another area of interest, since inflammatory zones are characterized by the production of hydroxyl radicals (ОН•). The effectiveness of ОН• interceptors in vivo depends on their ability to bind metals of variable valence. At the same time, SA has two binding sites that ensure the formation of the corresponding Fe3+/Cu2+ salicylate complex in the inflammation area. Thus, ASA and SA appear to be more effective than such well-established ОН• interceptors as ascorbate, glutathione, and cysteine. This is explained by two factors: high rate of ОН• reduction, and the ability to create high concentrations in areas of inflammation [10–12].
Although ASA possesses a broad range of positive influences on inhibiting the development of oxidative stress, inflammation, and thrombocyte aggregation, its use is limited due to the presence of adverse side effects. Like other NSAIDs, ASA can exert both local (irritation of the mucous membrane) and systemic (reducing the synthesis of prostaglandins) damaging effects on the gastrointestinal tract mucous membrane after oral administration, and may also impair liver and kidney functions [3, 4].
The presence of side effects makes it expedient to modify salicylates while maintaining their main therapeutic effects. One of the directions of research in increasing the effectiveness and lessening the side effects of ASA is the production of various derivatives of salicylic acid [4]. We hypothesized that the creation of a synthetic derivative of ASA and β-alanyl-L-histidine (carnosine), a natural antioxidant that has a broad spectrum of action [13, 14] and can prevent the formation of ulcers induced by the application of acetic acid [15], can improve the actions of both compounds, and attenuate the adverse effects. Disregarding the many positive actions of carnosine, and its lack of adverse side effects, its effectiveness is limited by both low lipophilicity and unavoidable hydrolysis, carried out by specific enzymes – serum and tissue carnosinases [16]. These features require the development of specialized medicine forms to protect carnosine from hydrolysis. The amino group of carnosine has been variously modified with the aim of obtaining carnosinase-resistant compounds with antioxidant activity. For example, carnosinase-resistant conjugates of carnosine with lipoic acid [17], Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) [18] and N-acetylcarnosine were created - the last was developed and successfully used in cataract treatment [19].
The creation of a carnosine conjugate with ASA could provide it protection from enzymes, increasing its bioavailability and improving its pharmacological characteristics, and simultaneously prevent adverse side effects of ASA on the gastrointestinal tract. The objective of our study was to obtain this conjugate, and to assess its biological activity - in particular, antioxidant, antiplatelet and anti-ulcer activities, as well as resistance to serum and tissue carnosinases.
Materials and methods
Chemistry
General methods
Acetylsalicylic acid (Hebei Jiheng (Group) Pharmaceutical Co., Ltd., China), carnosine (Hamari Chemicals, Japan); triethylamine, pivaloyl chloride (Fluka, Germany); acetonitrile, diethyl ether (Chimmed, Russia) were used in the synthesis of salicyl-carnosine. All solvents were anhydrous. Ticlopidine hydrochloride and Trolox ((±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) (Sigma Aldrich, USA) were used for the comparison of biological properties. Preparative purification of the resulting compound was carried out on a LiChroprep RP-18 reverse phase column (Merck Millipore, USA) (column diameter 30 mm, column height 245 mm, gradient 0–30% ethanol, flow rate 300 ml/h). Control of synthesis and preparative purification of the obtained compound was carried out using thin layer chromatography (TLC) on silica gel plates Silufol UV 254 (Kavalier, Czech Republic) with ninhydrin development. The solvent systems used and Rf values are presented in Table S1. Carnosine, acetylsalicylic (ASA) and salicylic acids (SA) were used as standards to identify the products of synthesis by Rf. The purity of the resulting compound was analyzed by means of high performance liquid chromatography (HPLC) on a MiliChrom-A02 chromatograph (Econova, Russia) with a spectrophotometric detector using a Prontosil 120 5C18aq reversed-phase column (75 × 2 mm) (BISCHOFF Chromatography, Germany). Analysis conditions: eluent A – 200 mM LiClO4 + 5 mM HClO4; eluent B – methanol; linear gradient 5–80% B for 16.5 min; flow rate 150 μl/min, column thermostat temperature − 30 °C; detector wavelength – 210 nm.
The spectral characteristic of the resulting compound was obtained at λ = 324 nm using an Ultrospec 3300 Pro spectrophotometer (Amersham Biosciences, USA). The starting and resulting compounds were dissolved in distilled water to a final concentration of 1 mM.
The melting point of the resulting compound was determined using a Boetius melting point apparatus (Boetius Franz-Kustner, Germany). The structure of the obtained compound was determined by mass spectrometry using the LCQ Advantage MAX system (Thermo Finnigan, USA) with electrospray ionization and syringe injection of samples at a concentration of 10 μg/ml in methanol.
1H NMR spectra were obtained using a Bruker Avance 300 spectrometer (Bruker Daltonics Inc., USA) at a frequency of 300 MHz. 13C NMR spectra were obtained using a Bruker Avance 600 spectrometer at a frequency of 150 MHz.
High-resolution mass spectra (HRMS) were measured on a Bruker micrOTOF II focus LC-MS system using electrospray ionization (ESI). The measurements were carried out in positive ionization mode (voltage at the capillary interface – 4500 V); range of mass scanning (m/z) varied from 50 to 3000 Da; both external and internal calibrations were performed using a standard Electrospray Calibrant Solution (Fluka, USA). Analyte solutions in acetonitrile, methanol or water were used for syringe administration (flow rate 3 ml/min). Nitrogen was used as a dehydrator; capillary interface temperature was 180 °C. Highly purified compound was obtained by HPLC using a Reprosil pur C18aq, 5 μm column (20 × 150 mm) (Dr. Maisch HPLC GmbH, Germany). Gradient elution was used: eluent A – methanol-water-acetic acid (30:70:0.1), eluent B – methanol; linear gradient 0–100% B for 20 min; flow rate 20 ml/min; detector wavelength – 226 nm.
Synthesis of 2-[3-(2-hydroxy-benzoylamino)-propionylamino]-3-(1H-imidazol-4-yl)-propionic acid (salicyl-carnosine)
2.0 g (11.1 mmol) of acetylsalicylic acid were dissolved in 40 ml of acetonitrile, cooled to −5 °C, then 1.76 ml (12.2 mmol) of triethylamine (TEA) were added to the solution and cooled to −30 °C, while stirring with a magnetic stirrer. 1.5 ml (12.2 mmol) of pivaloyl chloride were added to the cooled solution, stirred for 20 min at −10 °C with a magnetic stirrer, cooled to −30 °C, and mixed with a precooled carnosine solution. 2.5 g (11.1 mmol) of carnosine were dissolved in 7 ml of water and 14 ml of acetonitrile, 1.55 ml (11.1 mmol) of triethylamine were added, the mixture was cooled to −20 °C and mixed with the first solution 20 min later. The reaction mixture was held for 1 h at −10 °C and 12 h at 18–20 °C with magnetic stirring, and then evaporated on a rotary evaporator. The residue was dissolved in absolute methanol and precipitated with ethyl acetate. The resulting product was re-precipitated twice from absolute methanol with dry ether. The product was filtered by washing it repeatedly with dry ether on a filter [20].
The resulting compound was dried under vacuum over P2O5 to give 2.8 g of solid. According to the HPLC (LiChroprep RP-18 reverse phase column (Merck Millipore, USA), the content of the target compound in the precipitate equaled 22.3%. It was further purified by HPLC using a Prontosil 120 5C18aq reversed-phase column to give 96.7% pure target material (Fig. S1). MS-HPLC (m/z) [M-H] = 345 g/mol (Fig. S2).
Salicyl-carnosine was obtained as white powder: mp 167–168 °C, absorption wavelength λ = 324 nm (Fig. S3); 1H-NMR (300 MHz, D2O) δ 2.49–2.64 (m, 2H), 2.95–3.03 (m, 1H), 3.13–3.20 (m, 1H, J = 4.3 Hz), 3.51–3.63 (m, 2H), 4.48 (dd, 1H, J = 4.5 Hz), 6.90–6.97 (m, 2H), 7.06 (s, 1H), 7.41 (t, 1H, J = 7.7 Hz), 7.56 (d, 1H, J = 7.7 Hz), 8.19 (s, 1H) (Fig. S4); 13C-NMR (150 MHz, D2O) δ 28.0, 36.1, 36.7, 54.4, 116.9, 117.9, 120.8, 129.1, 130.5, 133.7, 134.9, 158.0, 158.3, 170.2, 174.2, 177.1 (Fig. S5); HRMS (m/z) [M + H] – calcd. For C16H18N4O5 347.1358; found 347.1350 (Fig. S6).
Biology
Ethics of experiments
Each human subject had given their written informed consent to blood sampling for research.
All manipulations with animals were carried out according to the Guide for the Care and Use of Laboratory Animals – Russian Version, National Academy Press, Washington, DC, 1996). All efforts were made to minimize animal suffering and to reduce the number of animals used.
All studies were approved by the Research Center of Neurology Ethics Committee, ethical permission number 2–5/19 from 20.02.2019.
The superoxide scavenging activity
The superoxide scavenging activity of complexes of carnosine and the obtained compound with copper and zinc was determined by the slowdown in nitro blue tetrazolium (NBT) reduction rate during the generation of superoxide anion radical in the process of xanthine oxidation with xanthine oxidase [21].
The reaction system for superoxide induction contained sodium carbonate buffer (50 mM, pH 10.2), 0.1 mM EDTA, 0.1 mM xanthine, and 25 mM NBT. To obtain the complexes, solutions of carnosine and SC were mixed with solutions of CuSO4 and ZnSO4 (all components at a final concentration of 100 mM) and incubated for 10 min. The resulting solution was added to cuvettes with the superoxide induction system at a final concentrations of 2, 4, and 10 mМ. The reaction was initiated by the addition of the enzyme.
SOD-like activity of the complexes was determined by the slowdown in the rate of optical density increase at 560 nm caused by the inhibition of NBT reduction, and was presented as percentage of the control samples value, which did not contain interceptors.
Аntioxidant activity determination by iron-induced chemiluminescence method
To assess the antioxidant activity of SC in vitro we used a model of lipid peroxidation (LPO) initiated by Fe2+ ions in isolated low density and very low density serum lipoproteins (LP) obtained from healthy donors [22]. The method is based on the measurement of chemiluminescence (CL) parameters in a suspension of LP under oxidation by ferrous ions added in excess to the reaction medium [23]. The following kinetic parameters of CL were measured: rapid CL flare amplitude (h, mV) reflecting the stationary level of lipid hydroperoxides, duration of CL ignition latent period (lag-period, τ, s) characterizing LP resistance to oxidation, which depends on the ratio of pro- and antioxidants in reactionary system, and the maximum intensity of chemiluminescence (H, mV) reflecting LP oxidation capacity.
SC antioxidant effect was compared to that of carnosine, ASA and Trolox (an antioxidant, soluble vitamin E analogue). The compounds were added to the reaction mixture as aliquots of 10 mM stock solutions (pH 7.45) along with a compensating volume of phosphate buffer to obtain a final concentration of 500 μM in a final volume of 0,9 ml.
The reaction was started by the addition of FeSO4 at a final concentration of 5 mM, and CL signal was recorded using a 1251 Luminometer (LKB-Wallac) at 37 °C while continuously stirring the solution, in 3 parallel samples for each compound (the mean value was used in the analysis).
The change in the studied parameters of the CL curve was expressed as a percentage of the control values in samples without test compounds.
Resistance to hydrolysis by tissue and serum carnosinases
Resistance of the obtained compound to hydrolysis by tissue and serum carnosinases (specific enzymes regulating the level of carnosine in the human and animal organisms and degrading it to β-alanine and L-histidine) was determined by estimating the concentration of histidine as a hydrolysis product, using the method proposed by Pegova et al. [24]. We used the dialysate of kidney tissue homogenate from 3 male Wistar rats as a source of tissue carnosinase. The experiment was conducted in accordance with ethics standards and the international requirements on laboratory animal handling. The enzyme was activated with MnCl2. To conduct the reaction, 20 μl of dialysate was added to 0.1 ml of the reaction sample (Tris-HCl buffer, 40 mM pH 8.0, 1 mM MnCl2) and incubated for 1 h at 37 °C. The hydrolysis reaction was initiated by the addition of substrate (carnosine or the obtained compound) at a final concentration of 10 mM. After 3 h, the reaction was stopped by adding 0.3 ml of cold 96% ethanol. The protein precipitate was removed by centrifugation for 10 min at 3000 g. The hydrolysis products in the supernatant were then determined. A mixture of serum samples obtained from several donors was used as a source of serum carnosinase. Serum carnosinase was activated by incubation with 5 mM CdCl2 at 37 °C for 30 min. Carnosine or SС were added to the samples at a concentration of 2 mM. Samples were incubated for 0, 30, 60, 120, and 180 min at 37 °C. Proteins were precipitated with 20% trichloroacetic acid (TCA), the precipitate was removed by centrifugation at 3500 g for 15 min, and the supernatant was used for analysis. Samples incubated without serum were used as the control. The content of SС and carnosine in the samples was determined by HPLC-mass spectrometry, as described above. The signal from the starting samples (0 min) containing carnosine and SС at a concentration of 2 mM in arbitrary units of integration was taken for 100%, the remaining samples were normalized to the peak area of the starting sample.
Luminol-dependent chemiluminescence response on BaSO4-activated respiratory burst of leukocytes
The model of luminol-dependent CL in human leukocytes was used for direct estimation of SC antioxidant activity in vitro. Leukocyte suspension was obtained from healthy donor heparinized blood (10 ml). Red blood cells underwent lysis by 5-fold volume hypotonic lysis solution (0,83% NH4Cl) and leukocytes were washed twice in phosphate buffer (2.7 mM KCl, 136.7 mM NaCl, 1.5 mM KH2PO4, pH = 8.1) and pelleted by centrifugation (500 g, 10 min, 4°С). Cleansed leukocytes were resuspended in Hanks’ Balanced Salt Solution (HBSS) (рН 7,4; final concentration 3–5·106 cells per 1 ml) and refrigerated. Samples for respiratory burst measurement were prepared in the same saline solution in the presence of 100 μM luminol. Final cell concentration in a sample was 5·105 cells/ml. After 1 min incubation at 37 °C, HBSS (as control) or the test compounds (diluted in HBSS) were added to the samples. After another minute CL response was induced by adding a suspension of BaSO4 (2 mg per sample). CL was registered using a 1251 Luminometer (LKB, Wallac) at 37 °C with constant stirring [25].
Oxidative hemolysis of red blood cells
The blood of healthy donors stabilized with heparin (20 U/ml) was used as a source of red blood cells. It was diluted 10 times with physiological saline (145 mM NaCl) and stored at 37 °C for no longer than 4 h. Resistance of red blood cells to oxidative stress was determined from hemolysis kinetics registered by the change in the optical density at 630 nm. The measurements were carried out at 37 °C with continuous stirring. A suspension of red blood cells was diluted to a concentration of (5 ± 0.3) · 106 cells / ml in a cuvette containing pyrophosphate buffer (10 mM, pH = 8.4, physiological solution). The cells were counted using a Goryaev’s chamber. Oxidative stress was induced by 0.2 mM sodium hypochlorite. The procedure was carried out until the maximum level of hemolysis was reached.
The effects of SC, carnosine, ASA, and Trolox on red cell oxidative hemolysis were evaluated by:
the decrease in hemolysis rate (v) determined from the slope of the tangent line;
the increase in time required for half of the red blood cells to undergo hemolysis in comparison to control (T 0.5).
To record hemolysis kinetics, test compounds were added to the cuvette at a final concentration of 0.1 mM before the oxidation inductor. The measured parameters were expressed as a percentage of the control values in samples without test compounds [26].
At the end of recording red blood cells hemolysis caused by hypochlorite, a control determination of the reaction mixture pH was carried out in all samples. pH control (8.3–8.4) was necessary because hypochlorite effectiveness decreases in an acidic environment.
An in vitro study on platelet aggregation
An in vitro experiment on platelet aggregation analysis in the presence of the obtained compound was carried out by the light transmission-aggregometry method using the physiological inductor ADP at a concentration of 10 μM. Other substances used for comparison were antiplatelet agents (acetylsalicylic acid and ticlopidine) and antioxidants (carnosine and Trolox). Platelets were obtained from donor blood (10 healthy individuals) according to a standard protocol. Quantitative measurement of platelet aggregation level was carried out by means of aggregometer graphical registration, optical density alterations were registered on a laser aggregometer (BIOLA Ltd., Russia) [27] via the turbidimetric method as described by Born G. (1961) [28].
Modeling of chronic gastric ulcer
Chronic gastric ulcer in rats was modeled according to the method proposed by Okabe et al. [29]. The experiments were carried out on 3 month old Wistar rats weighing 180–220 g. The animals were kept in a 12:12 h light-dark cycle at a temperature of 18–20 °C, with free access to food and water, according to generally accepted standards use of animals during experimental studies.
Ether anesthesia was used. For all operational manipulations, ether (3–5 ml per 1 kg of body weight) mixed with atmospheric air was applied through a mask. Before the surgery, rats were not fed for 18 h, while having free access to water.
The abdominal cavity was opened along the white line of the abdomen, and a ring with an inner diameter of 0.5 cm was placed on the serous membrane of the anterior wall of the stomach, with the application area chosen with the intent of avoiding large vessels. 0.03 ml of glacial acetic acid was added into the ring, and after 30 s, the remaining acid was removed with filter paper. The abdominal wall was closed layer by layer. Postoperative maintenance of the animals was carried out under normal conditions.
Four groups of 12 operated rats in each were used (Table 1). The first group (control) received saline (0.9% NaCl), the second – carnosine, the third – SС, and the fourth – ASA. Within each group, two different regimens of substance administration were tested. The substances were dissolved in saline and administered per os through a probe at a dose of 50 mg/kg body weight daily during either 1–5, or 1–10 days following the operation.
Table 1.
The distribution of animals into groups according to the regimen of administration of studied compounds
| Group number | Administered compound | Dose and group description | Regimen of administration (days after operation) |
|---|---|---|---|
| 1 | Saline (0,9% NaCl) | Control: 1.0 ml daily, per os, n = 12 | 1–5 |
| 1–10 | |||
| 2 | Carnosine | 50 mg/kg body weight daily, per os, n = 12 | 1–5 |
| 1–10 | |||
| 3 | Salicyl-carnosine (SC) | 50 mg/kg body weight daily, per os, n = 12 | 1–5 |
| 1–10 | |||
| 4 | Acetylsalicylic acid (ASA) | 50 mg/kg body weight daily, per os, n = 12 | 1–5 |
| 1–10 |
Statistical analysis
To determine the reliability of the differences Student’s t test was used. Differences were considered reliable at p < 0.05. The results are presented as the mean ± standard deviation (M ± SD) unless mentioned otherwise. Statistical evaluation of measuring ulcerative focus results was analyzed by factorial ANOVA followed by Tukey HSD test. Statistical processing was performed using “Statistica 13.0” software.
Results
Chemistry
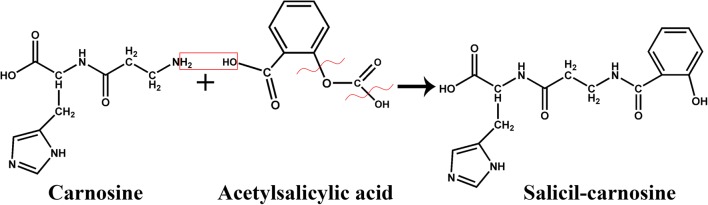
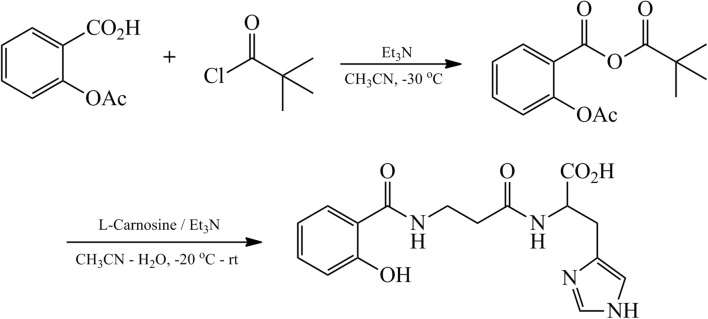
Synthesis of salicyl-carnosine is shown in Fig. 1. During the first step, ASA was activated by reacting with pivaloyl chloride in the presence of triethylamine in acetonitrile at −30 °C. The use of ASA, rather than salicylic acid, is necessary due to its high solubility in acetonitrile. Addition of the cooled carnosine aqueous acetonitrile solution to the prepared intermediate at the second step resulted in the formation of the target substance. SC was then purified by HPLC to obtain a sample of 96.7% purity that was then used in the biological experiments.
Fig. 1.
Scheme of salicyl-carnosine synthesis. 1 – Acetylsalicylic acid (ASA), 2 – pivaloyl chloride, 3 – salicyl-carnosine (SC); Et3N – trimethylamine, CH3CN – acetonitrile
Superoxide scavenging activity
Superoxide scavenging activity of carnosine and SC complexes with CuSO4 and ZnSO4 appeared to be concentration-dependent. These complexes almost completely inhibited NBT reduction at 10 mM (100% inhibition). SC has appeared to be 2.5 times more effective than carnosine at 4 mM (level of inhibition by SC – 69.2 ± 3.0%; by carnosine – 27.5 ± 2.6%, p < 0.001), while at 2 mM the superoxide scavenging activity of both compounds was similarly low (approximately 10%) (Fig. 2).
Fig. 2.
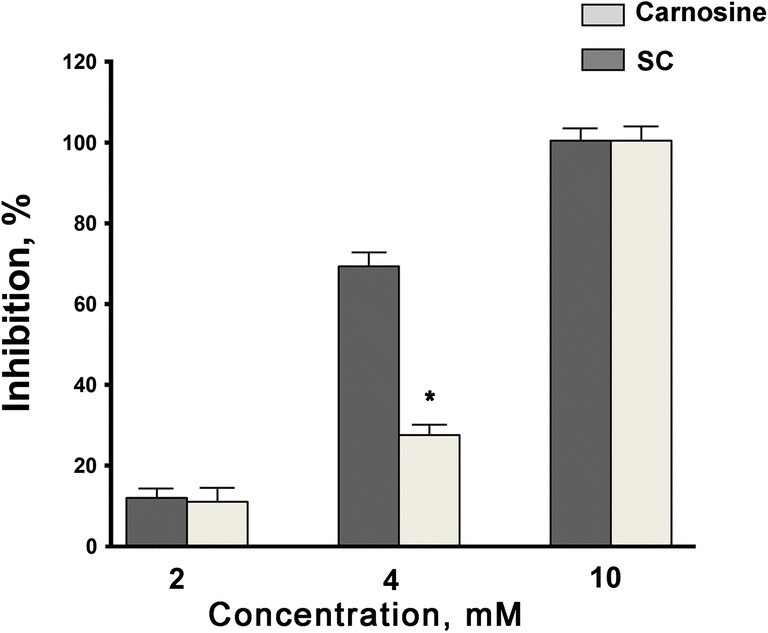
Slowdown of nitro blue tetrazolium reduction rate in the xanthine/xanthine oxidase superoxide radical induction system caused by carnosine and salicyl-carnosine (SC) used at concentrations of 2, 4 and 10 mM (* – p < 0.01 compared to carnosine, t-test). N = 10 measurements on each concentration
Chemiluminescence measurement of SC antioxidant activity
The effects of test compounds on CL parameters are shown in Table 2. The effect of the pre-formed lipoperoxides on oxidation for carnosine and SC was found to be comparable to that of Trolox: the intensity of rapid CL flare is reduced by 46%, 43% and 47% (p < 0.05), respectively (parameter h in the table). The duration of the oxidation lag phase is increased by 220%, 190% and 290% (p < 0.001) for carnosine, SC and Trolox, respectively (parameter τ in table). In addition, this parameter for SC was more than two times higher than for ASA. The ability of studied compounds to inhibit rapid CL flare development suggests their interaction with pre-existing hydroperoxides in the model system, while the increase in the CL latent period confirms their direct action as intercepting antioxidants. Moreover, SC suppresses the oxidation of LP (maximum intensity of CL, parameter H in table) more efficiently than carnosine. At the same time, ASA has shown lower antioxidant activity in comparison to carnosine and SC, while Trolox has retained the greatest effect, known as “gold standard” in the study of the activity of natural antioxidants.
Table 2.
Changes in the parameters of iron-induced CL caused by the carnosine, salicyl-carnosine (SC), acetylsalicylic acid (ASA) and Trolox (final concentration 500 μM)
| Compound | CL parameter (% from control) | ||
|---|---|---|---|
| h | τ | H | |
| Carnosine | 54.0 ± 2.5* | 320.0 ± 7.7* | 92.0 ± 4.0 |
| SC | 57.0 ± 5.5* | 290.0 ± 6.1*# | 78.0 ± 3.2*# |
| ASA | 70.0 ± 4.8*# | 125.0 ± 2.1*# | 89.0 ± 3.4* |
| Trolox | 53 ± 1* | 390 ± 5.1*# | 8,5 ± 1*# |
Results are expressed as mean ± SEM. * – p < 0.05 as compared to control, # – p < 0.05 as compared to carnosine, t-test
Resistance to hydrolysis by tissue and serum carnosinases
We evaluated SC’s resistance to hydrolysis by tissue and serum carnosinases. Resistance of SC and free carnosine to hydrolysis by tissue carnosinase was determined by estimating the concentration of histidine as a hydrolysis product. Carnosine was completely hydrolyzed by the tissue carnosinase in 1 h, whereas SC did not undergo hydrolysis for three hours (Table 3).
Table 3.
Rate of carnosine and salicyl-carnosine hydrolysis carried out by rat kidney carnosinase after 1 h of incubation
| Substrate (10 mM) | Accumulation of histidine (μmol/g of tissue per hour) | Relative hydrolysis rate (%) |
|---|---|---|
| Carnosine | 31.6 ± 3.2 | 100.0 ± 2.4 |
| Salicyl-carnosine (SC) | 0 ± 0* | 0 ± 0* |
*– p < 0.001 as compared to carnosine. N = 24 (8 parallel samples of kidney dialysates from 3 rats)
The MS-HPLC method was used to determine the resistance of obtained compounds to the serum carnosinase - the dynamics of decrease in amounts of both substrates were measured. Carnosine was hydrolyzed with the serum carnosinase by 86% in 1 h and completely in 2 h, whereas SС did not undergo hydrolysis over the course of 3 h (Fig. 3).
Fig. 3.
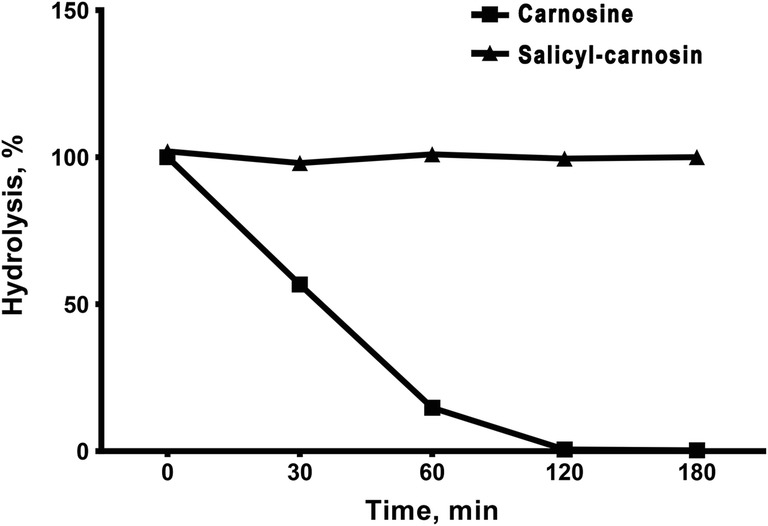
Kinetics of SC and carnosine hydrolysis by serum carnosinase during 180 min. N = 6 samples on each time point
Thus, salicylic acid being attached to the alanine fragment has completely protected the carnosine derivative from hydrolysis, which increased its lifespan within the organism.
Antioxidant activity of tested compounds during BaSO4-activated respiratory burst of leukocytes
Trolox is known to be an effective ROS scavenger [30] and was therefore selected as a reference compound in this study. We first investigated the influence of Trolox in different concentrations on CL response of activated leukocytes. The concentration of Trolox that caused 51.9 ± 0.9% (p < 0.001) inhibition of CL response (100 μM) was chosen. Next the influences of carnosine, SC and ASA at the same concentration were studied.
The effects of tested compounds on luminescence response value are shown in Fig. 4. SC decreases BaSO4-induced CL to 76.8 ± 2.7% (p < 0.01), but not as effectively as Trolox does. At the same time, CL inhibiting effect was more expressed in SC than in its precursors carnosine (90.1 ± 4.1, p = 0.05) and ASA (98.4 ± 5.6, p = 0,29).
Fig. 4.
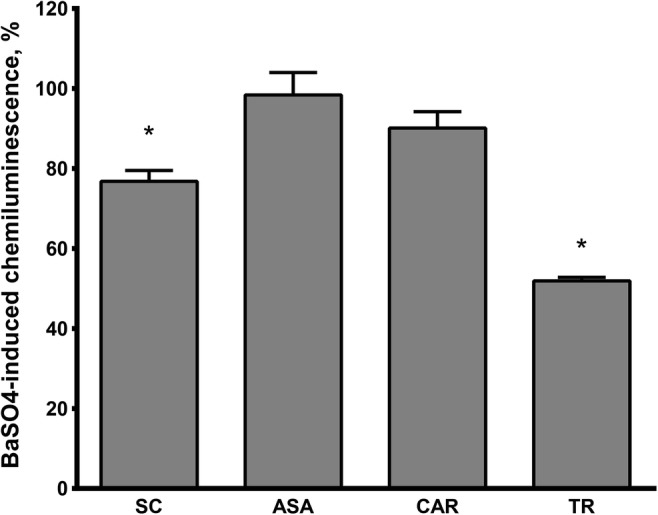
Comparison of Trolox (TR), carnosine (CAR), SC and ASA effects on luminol-enhanced chemiluminescence of leukocytes induced by BaSO4. The data are presented as percentage from control value without tested substances. (* – significant differences with p < 0.05 compared to control, t-test). N (in each group) = 10
Red blood cell structure stabilization under oxidative stress
Carnosine was used as a reference substance in studying SC antihemolytic activity. It was shown in a preliminary experiment that 50% hemolysis inhibition is provided by carnosine at a concentration of 25 μM. An equivalent concentration of SC, ASA and Trolox was used to evaluate their antihemolytic effect. The results of these studies are presented in Table 4.
Table 4.
Effects of carnosine, salicyl-carnosine (SC), acetylsalicylic acid (ASA) and Trolox (final concentration 25 μM) on kinetic parameters of red blood cells hemolysis caused by sodium hypochlorite (data presented as percentages of the control value without substances)
| Compound | V of hemolysis, % from control | T0.5 of hemolysis, % from control |
|---|---|---|
| Carnosine | 50 ± 4* | 223 ± 14* |
| SC | 48 ± 3* | 227 ± 24* |
| ASA | 100 ± 7 | 99 ± 10 |
| Trolox | 82 ± 8* | 126 ± 27 |
*– p < 0.05 compared to control
All of the tested compounds did not induce hemolysis themselves. However, they slowed down the rate of oxidative hemolysis of human red blood cells. SC and carnosine at a concentration of 25 μM demonstrated a strong and comparable inhibitory effect on red blood cell hemolysis caused by sodium hypochlorite, reducing hemolysis rate to 50 ± 4% and 48 ± 3% (p < 0.001), respectively, and increasing the time required for half of the red blood cells to undergo hemolysis to 227 ± 24% and 223 ± 14% (p < 0.001), respectively. ASA did not influence the measured parameters under these conditions. Trolox did not have an influence on hemolysis inhibition halftime (reducing it to 82 ± 8%, p < 0.05) and have a slight effect on hemolysis rate (increasing it to 126 ± 27%, p = 0.07).
An in vitro study on antiplatelet activity
After obtaining platelets from donors’ venous blood according to standard procedure, antiplatelet activity of compounds was estimated. Aggregation of platelets was initiated by the addition of ADP solution. We used ASA as a test compound, since it is known to be an effective antiplatelet drug. At first, ASA concentration that would provide 50% inhibition under standard conditions was estimated (Table 5).
Table 5.
Effect of acetylsalicylic acid (ASA) in various concentrations on ADP-induced platelet aggregation (data presented as percentages of the control value without ASA)
| ASA concentration, μM | Platelet aggregation (%) |
|---|---|
| 8.5 | 92.4 ± 11.2 |
| 85 | 52.5 ± 6.1* |
| 150 | 65.4 ± 7.5* |
| 500 | 38.1 ± 2.9# |
* – p < 0.05, # – p < 0.01 as compared to control, t-test, N = 10
For further studies 85 μM ASA (reducing aggregation to 52.5 ± 6.1%, p < 0.001) was used to compare the antiplatelet effect of SC with the corresponding ability of other drugs at the same concentration. As it is demonstrated in Fig. 5, experiments on blood samples of 10 healthy subjects show that SC inhibited platelet aggregation as effectively as ticlopidine (a standard antiplatelet agent) and one of its precursors ASA and reduced it to 58.1 ± 6.9%, p < 0.001. At the same time, SC demonstrated a more prominent effect than its other precursor, carnosine (77.3 ± 5.5, p < 0.05). Trolox had no effect on platelet aggregation.
Fig. 5.
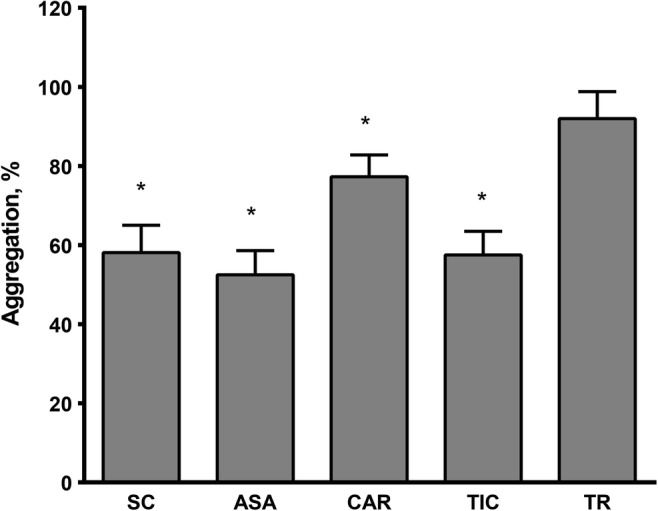
The influence of salicyl-carnosine (SC), acetylsalicylic acid (ASA), carnosine (CAR), ticlopidine (TIC) and Trolox (TR) on ADP-induced platelet aggregation (the data are presented as percentages of the control value without tested substances, M ± SEM); (* – p < 0.05 as compared to control, t-test). N (in each group) = 10
Chronic gastric ulcer lesion impediment
The administration of carnosine and SC at the stage of mucosal lesion formation and subsequent epithelialization provided an effective reduction of the lesion area compared to the control animals, which did not receive the studied compounds (Fig. 6). Factorial ANOVA showed the significant effect of injected substances in the experimental groups [F (3,35) = 87.44, p < 0.0001] and a factor of administration regimen - 5 or 10 days was discovered [F (1,35) = 301.37, p < 0.0001].
Fig. 6.
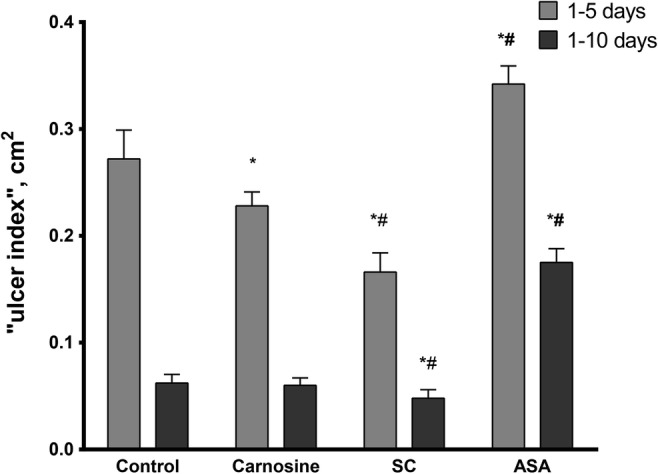
Effect of carnosine, salicyl-carnosine (SC), and acetylsalicylic acid (ASA) on the healing of acetate ulcers on the anterior wall of the stomach in rats (* – p < 0.05 as compared with control; # – p < 0.05 as compared with carnosine). N (in each group) = 6. Data statistics were analyzed using factorial ANOVA followed by Tukey HSD test
At the stage of mucosal lesion formation (1–5 days after the operation) in animals treated orally through a probe with SC or carnosine, at a daily dose of 50 mg/kg body weight, the lesion area was 61.0 ± 6.6%, p < 0.001 and 83.8 ± 4.8%, p < 0.02 respectively, as compared to the saline-treated control group taken for 100%. By the end of the experiment, on the 10th day of compound administration (epithelialization stage), the lesion area in the carnosine-treated rats amounted to 96.5 ± 11.2%, p = 0.95 of the control, and 77.2 ± 12.9%, p < 0.05 for the rats treated with SC. Thus, SC contributes to a more rapid healing of the ulcer.
The administration of ASA at an early stage (1–5 days) has led to an increase of the lesion area by 25.7 ± 6.2%, p < 0.002 as compared to the control animals, while suppressing the healing process and preventing epithelization of the damaged gastric mucosa at subsequent stages. Near the end of experiment, the lesion area was 3.9 times more than the corresponding area of the ulcer in the control group (281.3 ± 20.9%, p < 0.001).
Discussion
A novel derivative of carnosine and ASA, salicyl-β-alanyl-L-histidine (salicyl-carnosine, SC), was obtained by coupling of an antiplatelet agent ASA with a natural antioxidant L-carnosine. In order to perform antioxidant properties of new compound, first we analyzed its SOD-like activity. It is known that Cu/Zn-SOD carries Cu and Zn in its active center, where these ions are coordinated to histidine [31]. It has been shown that carnosine complexes with zinc and copper are effective superoxide scavengers, that play an essential role in the antioxidant effect of this dipeptide in vivo [21]. Besides, carnosine derivatives are also suggested to manifest SOD-like activity. There are experimental data testifying the ability of SC – Cu/Zn ion complexes to demonstrate an effective superoxide scavenging activity. The resulting product demonstrated 2.5 greater SOD-like activity than carnosine. On one hand, SOD-like activity of SC is comparable to that of ASA [32], on the other, there is data showing that high doses of ASA can impair the antioxidant defenses of the cell [33]. SOD-like activity was also identified for copper-carnosine, copper-anserine and copper-homocarnosine complexes [34]. For ASA derivatives is also known, that 5-aminosalicylic acid have antioxidant activity [35, 36].
Furthermore, CL parameters of iron-induced lipoprotein peroxidation of SC revealed its high antioxidant activity. Antioxidant activity of SC in iron-induced lipoprotein peroxidation was comparable or exceeded the action of those of carnosine and ASA. Previously, together with “Hamari Chemicals, Ltd”, we synthesized several derivatives of carnosine with Trolox: (S)-Trolox-L-carnosine (STC), and (R)-Trolox-L-carnosine (RTC). It was shown that STC, RTC and Trolox suppressed oxidative hemolysis of red blood cells less efficiently than carnosine when taken in the same concentration. Both STC and RTC were resistant toward hydrolytic degradation by the human serum carnosinase. STC and RTC were concluded to demonstrate higher antioxidant capacity in the iron induced-CL model than their precursors, carnosine and Trolox [18]. The carnosine derivative SC, investigated in this study, is resistant to hydrolysis by tissue and serum carnosinases, possesses a high level of antioxidant activity, and effectively suppresses oxidative hemolysis of red blood cells on a level comparable with carnosine. The carnosinase-resistance improved the bioavailability of carnosine and increased the duration of its pharmacological action in the organism.
Polymorphonuclear leukocytes are the first cells to appear at the site of an acute inflammatory response and have been implicated in a number of pathological processes involving inflammatory reactions (such as the period following a stroke). These cells, when stimulated, produce reactive oxygen metabolites (e.g. superoxide, hydroxyl radical, hydrogen peroxide and hypochlorite) [37]. This process is accompanied by increased consumption of molecular oxygen. Under such conditions, in the pharmacological treatment of chronic inflammatory disorders it is of interest to use NSAIDs and antioxidants that are able to react directly with these metabolites or to inhibit their generation in the respiratory burst by specific mechanisms, in addition to NSAID’s classical inhibition of cyclooxygenase [38]. Carnosine and other compounds containing imidazole and free amine sites have been proposed as protective agents against hypochlorous acid-mediated damage and may be able to limit hypochlorous acid-mediated oxidation in leukocytes [39]. SC at 0.1 mM decreases CL response of activated leukocytes but not as effectively as Trolox does. At the same time, the CL inhibiting effect was more expressed in SC than in either of its precursors. Banbarek et al shown that ASA generate inhibition of luminal CL modulated by phorbol 12-myristate 13-acetate in neutrophils of horses which is dose-dependent (at concentrations 0.1–5 mM), but at the concentration of 0.1 mM it is not significant [40]. In present study, carnosine’s inhibiting action at 0.1 mM was weak and ASA had no effect at all. Earlier we have shown that carnosine at higher concentrations (1.0–4.0 mM) suppresses luminol-dependent CL response to BaSO4-activated respiratory burst of leukocytes on 36–61% [41]. Thus, SC proved to be effective at a lower concentration than carnosine and ASA.
One of the aims of this research is to avoid the derivative exhibiting a hemolytic effect. Furthermore, it is of interest to make a substance that will possess antihemolytic effects. Red blood cells are a convenient cellular model for studying oxidative damage mechanisms - oxidative hemolysis of red blood cells is widely used to evaluate antioxidant activity of natural antioxidants and chemical compounds [42]. In our study, SC’s ability to stabilize red blood cell structure during oxidative damage caused by sodium hypochlorite was comparable to that of carnosine. ASA did not affect any of the hemolysis parameters.
We proved, that the antioxidant properties of SC are comparable to carnosine, and this provided a possibility to test the antiplatelet action characteristic of its precursor ASA. Analysis of platelet function, which is widely used for diagnostic work-up in patients with predisposition to thrombosis and cerebrovascular diseases, including evaluation of the antithrombotic therapy effectiveness [43], revealed that SC has inhibited platelet aggregation more effectively compared to carnosine and was comparable in effectiveness with ASA and another antiplatelet agent ticlopidine. Previously, it has been shown that carnosine exerts a modulating effect by decreasing high and increasing low platelet aggregation, while having no effect in more than 50% of cases. Under the joint action of carnosine and ASA, mutual enhancement of effects was not found [44]. A more detailed study of the antiaggregation activity of SC in the blood of patients with high, normal and low rate of aggregation will be carried out at the next stage of the research.
One of the important side effects of ASA is the development of gastric ulceration and erosion [45]. As consequence, its modifications with a lessened, or absent, effect are in development. A novel ASA derivative with nano-hydroxyapatite (a kind of inorganic particle containing Ca2+), possessing anti-thrombotic properties comparable to ASA, and having much lower gastric mucosal damage than ASA, has been described [46]. It is known that carnosine possesses an anti-ulcer effect both on its own [15] and in bound form. Polaprezinc, a complex of carnosine with zinc, proved its protective effect against gastric damage induced by immersion stress, ischemia/reperfusion, ASA, ethanol, or histamine in many experimental models [47–50]. ASA is capable of inducing gastric damage, while carnosine has a reverse effect. Due to these properties, an investigation of the anti-ulcer action of SC was proposed, which has shown that SC not only lacked a negative effect on the acetic acid induced gastric ulcer model in rats, but protected the gastric mucosa against the formation of the stomach ulcerative lesions and promoted their epithelization, therefore overcoming this highly undesirable inherent side effect of ASA.
Conclusion
The biological evaluation of the derivative (salicyl-carnosine, SC) of carnosine and ASA revealed that SC has retained pharmacologically significant properties of its precursors (antioxidant and antiplatelet effects) but lacked ASA side effect (stomach ulceration) and was resistant to specific hydrolysis by serum and tissue carnosinases. The obtained results emphasize the importance of further study of the mechanisms of salicyl-carnosine action, its possible neuroprotective and anti-inflammatory properties and creating an antithrombotic drug based on it.
Electronic supplementary material
(DOCX 1199 kb)
Acknowledgments
This work was supported by state assignment of Research Center of Neurology.
Abbreviations
- ASA
acetylsalicylic acid
- CL
chemiluminescence
- COX
cyclooxygenase
- HPLC
high performance liquid chromatography
- HRMS
high resolution mass spectrometry
- LP
lipoproteins
- LPO
lipid peroxidation
- NBT
nitroblue tetrazolium
- NSAIDs
non-steroidal anti-inflammatory drugs
- ROS
reactive oxygen species
- SA
Salicylic acid
- SC
salicyl-carnosine
- SOD
Cu/Zn-superoxide dismutase
- TEA
trimethylamine
- TLC
thin layer chromatography
- TCA
trichloroacetic acid
- STC
Trolox-L-carnosine
- RTC
(R)-Trolox-L-carnosine
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vlot AC, Dempsey DA, Klessig DF. Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- 2.Vane J. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 3.Awtry EH, Loscalzo J. Aspirin. Circulation. 2000;101:1206–1218. doi: 10.1161/01.CIR.101.10.1206. [DOI] [PubMed] [Google Scholar]
- 4.Ekinci D. Salicylic acid derivatives: synthesis, features and usage as therapeutic tools. Expert Opin Ther Pat. 2011;21:1831–1841. doi: 10.1517/13543776.2011.636354. [DOI] [PubMed] [Google Scholar]
- 5.Mekaj YH, Daci FT, Mekaj AY. New insights into the mechanisms of action of aspirin and its use in the prevention and treatment of arterial and venous thromboembolism. Ther Clin Risk Manag. 2015;11:1449–1456. doi: 10.2147/TCRM.S92222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domashenko MA, Maksimova MY, Tanashyan MM. Acetylsalicylic acid in the treatment and prevention of cerebrovascular diseases. RMJ Neurol Psychiatry. 2011;30:1930–6 Russian.
- 7.Mitchell AG, Broadhead JF. Hydrolysis of solubilized aspirin. J Pharm Sci. 1967;56:1261–1266. doi: 10.1002/jps.2600561009. [DOI] [PubMed] [Google Scholar]
- 8.Rowland PM, Riegelman S, Harris PA, Sholkoff SD, Eyring EJ. Kinetics of acetylsalicylic acid disposition in man. Nature. 1967;215:413–414. doi: 10.1038/215413a0. [DOI] [PubMed] [Google Scholar]
- 9.Tanashyan MM, Domashenko MA, Raskurazhev AA. Aspirin resistance: clinical and molecular genetic evaluation techniques. Annals Clin Exp Neurol. 2016;10:41–6 Russian.
- 10.Shi X, Ding M, Dong Z, Chen F, Ye J, Wang S, Leonard SS, Castranova V, Vallyathan V. Antioxidant properties of aspirin: characterization of the ability of aspirin to inhibit silica-induced lipid peroxidation, DNA damage, NF-kappaB activation, and TNF-alpha production. Mol Cell Biochem. 1999;199:93–102. doi: 10.1023/A:1006934612368. [DOI] [PubMed] [Google Scholar]
- 11.Aruoma OI, Halliwell B. The iron-binding and hydroxyl radical scavenging action of anti-inflammatory drugs. Xenobiotica. 1988;18:459–470. doi: 10.3109/00498258809041682. [DOI] [PubMed] [Google Scholar]
- 12.Baltazar MT, Dinis-Oliveira RJ, Duarte JA, Bastos ML, Carvalho F. Antioxidant properties and associated mechanisms of salicylates. Curr Med Chem. 2011;18:3252–3264. doi: 10.2174/092986711796391552. [DOI] [PubMed] [Google Scholar]
- 13.Berezhnoy DS, Stvolinsky SL, Lopachev AV, Devyatov AA, Lopacheva OM, Kulikova OI, Abaimov DA, Fedorova TN. Carnosine as an effective neuroprotector in brain pathology and potential neuromodulator in normal conditions. Amino Acids. 2019;51(1):139–150. doi: 10.1007/s00726-018-2667-7. [DOI] [PubMed] [Google Scholar]
- 14.Boldyrev AA, Aldini G, Derave W. Physiology and pathophysiology of Carnosine. Physiol Rev. 2013;93:1803–1845. doi: 10.1152/physrev.00039.2012. [DOI] [PubMed] [Google Scholar]
- 15.Trubitsina IE, Shabanova ME, Chikunova BZ, Shavratsky VK, Formazyuk VB, Sergienko VI, Stvolinsky SL, Boldyrev AA. Characteristics of antiulcer activity of carnosine. Patol Fiziol Eksp Ter. 1997;4:17–20. [PubMed] [Google Scholar]
- 16.Wolos A, Piekarska K. Carnosinase in the kidney and liver. Int J Biochem. 1975;6:723–6.
- 17.Stvolinsky SL, Antonova NA, Kulikova OI, Lopachev AV, Abaimov DA, Al-Baidani I, Lopacheva OM, Fedorova TN, Kaplun AP, Sorokoumova GM. Lipoilcarnosine: synthesis, study of physico-chemical and antioxidant properties, biological activity. Biomed Khim. 2018;64(3):268–275. doi: 10.18097/PBMC20186403268. [DOI] [PubMed] [Google Scholar]
- 18.Stvolinsky SL, Bulygina ER, Fedorova TN, Meguro K, Sato T, Tyulina OV, Abe H, Boldyrev AA. Biological activity of novel synthetic derivatives of carnosine. Cell Mol Neurobiol. 2010;30(3):395–404. doi: 10.1007/s10571-009-9462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubois VD, Bastawrous A. N-acetylcarnosine (NAC) drops for age-related cataract. Cochrane Database Syst Rev. 2017;28;2:CD009493. 10.1002/14651858.CD009493.pub2. [DOI] [PMC free article] [PubMed]
- 20.Tanashyan MM, Fedorova TN, Stvolinskij SL, Andreeva LA, Nagaev IY, Migulin VA, Shabalina AA, Trubitsyna IE, Lopachev AV, Kulikova OI, Abaimov DA. Agent possessing antiaggregant, cytoprotective and antioxidant activity. RU 2694061 C1.
- 21.Gulyaeva NV. Superoxide-scavenging activity of carnosine in the presence of copper and zinc ions. Biokhimiia. 1987;52(7):1216–1220. [PubMed] [Google Scholar]
- 22.Fedorova TN, Us KS, Ostrovskaya RU. Estimation of the antioxidant effect of the Nootropic dipeptide Noopept on the model of Fe2+-induced Chemiluminescence of lipoproteins of human serum in vitro. Neurochem J. 2007;1:260–263. doi: 10.1134/S1819712407030154. [DOI] [Google Scholar]
- 23.Vladimirov YA. Studies of the antioxidant activity by measuring chemiluminescence kinetics. in Proceedings of the International Symposium on Natural Antioxidants: Molecular Mechanism and Health Effects / Ed. Packer L., Traber M.G., Xin W. AOCS Press: Champaing., Illinois, 1996:125–144.
- 24.Pegova A, Abe H, Boldyrev A. Hydrolysis of carnosine and related compounds by mammalian carnosinases. Comp Biochem Physiol. Part B. Biochem Mol Biol. 2000;127:443–446. doi: 10.1016/S0305-0491(00)00279-0. [DOI] [PubMed] [Google Scholar]
- 25.Boldyrev A, Abe H, Stvolinsky S, Tyulina O. Effects of carnosine and related compounds on generation of free oxygen species: a comparative study. Comp Biochem Physiol B Biochem Mol Biol. 1995;112(3):481–485. doi: 10.1016/0305-0491(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 26.Prokopieva VD, Bohan NA, Johnson P, Abe H, Boldyrev AA. Effects of carnosine and related compounds on the stability and morphology of erythrocytes from alcoholics. Alcohol Alcohol. 2000;35:44–48. doi: 10.1093/alcalc/35.1.44. [DOI] [PubMed] [Google Scholar]
- 27.Tanashyan MM, Spavronskaya LR, Shabalina AA, Abaimov DA, Raskurazhev AA. Platelet aggregation characteristics and pharmacokinetics of salicylic and acetylsalicylic acids in patients with cerebrovascular disorders. Eksp Klin Farmakol. 2017;80:48–51. [Google Scholar]
- 28.Born GVR. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1961;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- 29.Okabe S, Roth JL, Pfeiffer CJ. A method for experimental, penetrating gastric and duodenal ulcers in rats. Observations on normal healing. Am J Dig Dis. 1971;16:277–284. doi: 10.1007/BF02235252. [DOI] [PubMed] [Google Scholar]
- 30.Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic Biol Med. 1993;14(3):303–311. doi: 10.1016/0891-5849(93)90027-R. [DOI] [PubMed] [Google Scholar]
- 31.Fee JA, Peisach J, Mims WB. Superoxide dismutase. Examination of the metal binding sites by electron spin echo spectroscopy. J Biol Chem. 1981;256(4):1910–1914. [PubMed] [Google Scholar]
- 32.Kataoka M, Tonooka K, Ando T, Imai K, Aimoto T. Hydroxyl radical scavenging activity of nonsteroidal anti-inflammatory drugs. Free Radic Res. 1997;27(4):419–427. doi: 10.3109/10715769709065781. [DOI] [PubMed] [Google Scholar]
- 33.Durak I, Karaayvaz M, Cimen MY, Avci A, Cimen OB, Büyükkoçak S, Oztürk HS, Ozbek H, Kaçmaz M. Aspirin impairs antioxidant system and causes peroxidation in human erythrocytes and Guinea pig myocardial tissue. Hum Exp Toxicol. 2001;20(1):34–37. doi: 10.1191/096032701674627721. [DOI] [PubMed] [Google Scholar]
- 34.Kohen R, Misgav R, Ginsburg I. The SOD like activity of copper:carnosine, copper:anserine and copper:homocarnosine complexes. Free Radic Res Commun. 1991;12–13 Pt 1:179–85. [DOI] [PubMed]
- 35.Joshi R, Kumar S, Unnikrishnan M, Mukherjee T. Free radical scavenging reactions of sulfasalazine, 5-aminosalicylic acid and sulfapyridine: mechanistic aspects and antioxidant activity. Free Radic Res. 2005;39(11):1163–1172. doi: 10.1080/10715760500177880. [DOI] [PubMed] [Google Scholar]
- 36.Couto D, Ribeiro D, Freitas M, Gomes A, Lima JL, Fernandes E. Scavenging of reactive oxygen and nitrogen species by the prodrug sulfasalazine and its metabolites 5-aminosalicylic acid and sulfapyridine. Redox Rep. 2010;15(6):259–267. doi: 10.1179/135100010X12826446921707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Badwey JA, Karnovsky ML. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- 38.Black HS. Role of reactive oxygen species in inflammatory process. In: Hensbyand C., Lowe NJ, editors. Non-steroidal Anti-inflammatory Drugs: Pharmacology and the Skin. Basel: Karger;1989. Vol. 2, p. 1–20.
- 39.Pattison DI and Davies MJ. Evidence for rapid inter- and intramolecular chlorine transfer reactions of histamine and carnosine chloramines: Implications for the prevention of hypochlorous acid-mediated damage. Biochemistry. 2006;4;45(26):8152–8162. [DOI] [PubMed]
- 40.Benbarek H, Ayad A, Deby-Dupont G, Boukraa L, Serteyn D. Modulatory effects of non-steroidal anti-inflammatory drugs on the luminol and lucigenin amplified chemiluminescence of equine neutrophils. Vet Res Commun. 2012;36(1):29–33. doi: 10.1007/s11259-011-9507-5. [DOI] [PubMed] [Google Scholar]
- 41.Stvolinsky SL, Sousa Pontesh E, Sergienko VI. Boldyrev AA Immunomodulatory effects of carnosine in vitro and in vivo. Biol Membr. 1996;13(3):299–306. [Google Scholar]
- 42.Fedorova TN, Belyaev MS, Trunova OA, Gnezditsky VV, Maximova MY, Boldyrev AA. Neuropeptide carnosine increases stability of lipoproteins and red blood cells as well as efficiency of immune competent system in patients with chronic discirculatory encephalopathy. Biochem (Moscow) Suppl Ser A. 2009;3:62–65. doi: 10.1134/S1990747809010085. [DOI] [Google Scholar]
- 43.Yeung J, Li W, Holinstat M. Platelet signaling and disease: targeted therapy for thrombosis and other related diseases. Pharmacol Rev. 2018;70(3):526–548. doi: 10.1124/pr.117.014530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikitenko NI, Shavratskiĭ VK, Boldyrev AA, Suslina ZA, Ionova VG. Effect of carnosine and its derivatives on ADP-induced human platelet aggregation. Biomed Khim. 1995;41(1):41–3 Russian. [PubMed]
- 45.Ukawa H, Yamakuni H, Kato S, Takeuchi K. Effects of cyclooxygenase-2 selective and nitric oxide-releasing nonsteroidal anti-inflammatory drugs on mucosal ulcerogenic and healing responses of the stomach. Dig Dis Sci. 1998;43:2003–2011. doi: 10.1023/A:1018846912032. [DOI] [PubMed] [Google Scholar]
- 46.Zhen XE, Zong M, Gao SN, Cao YG, Jiang L, Chen SX, Wang K, Sun SQ, Peng HS, Bai YH, Li S. Preparation and characterization of a novel aspirin derivative with anti-thrombotic and gastric mucosal protection properties. PLoS One. 2014;3;9(6):e98513. doi: 10.1371/journal.pone.0098513. [DOI] [PMC free article] [PubMed]
- 47.Naito Y, Yoshikawa T, Yagi N, et al. Effects of polaprezinc on lipid peroxidation, neutrophil accumulation, and TNF-alpha expression in rats with aspirin-induced gastric mucosal injury. Dig Dis Sci. 2001;46(4):845–851. doi: 10.1023/A:1010716804594. [DOI] [PubMed] [Google Scholar]
- 48.Yoshikawa T, Naito Y, Tanigawa T, Yoneta T, Yasuda M, Ueda S, Oyamada H, Kondo M. Effect of zinc-carnosine chelate compound (Z-103), a novel antioxidant, on acute gastric mucosal injury induced by ischemia-reperfusion in rats. Free Radic Res Commun. 1991;14(4):289–296. doi: 10.3109/10715769109088958. [DOI] [PubMed] [Google Scholar]
- 49.Ueda K, Ueyama T, Oka M, et al. Polaprezinc (zinc L-carnosine) is a potent inducer of anti-oxidative stress enzyme, heme oxygenase (HO)-1—a new mechanism of gastric mucosal protection. J Pharmacol Sci. 2009;110(3):285–294. doi: 10.1254/jphs.09056fp. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Q, Feng L. Protective effect of polaprezinc on acute gastric mucosal injury in rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2019;28;44(1):22–27. 10.11817/j.issn.1672-7347.2019.01.004. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 1199 kb)