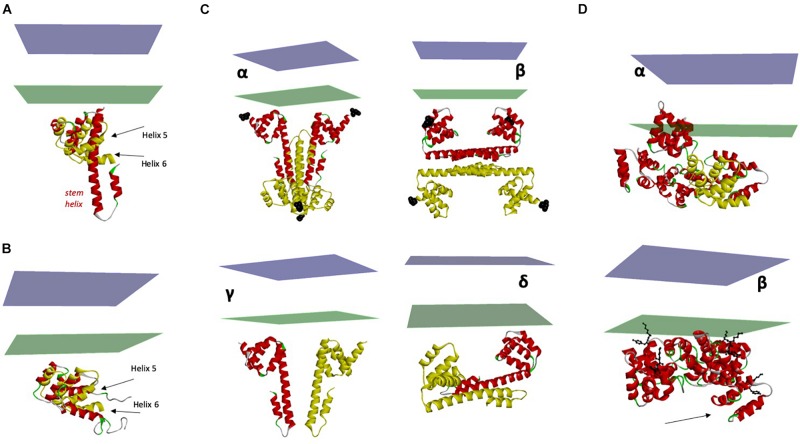
FIGURE 5.
Structure and flexibility of CD95 ICD. (A) Superimposition of the two crystal structures of CD95 death domain (PDB:3EZQ in red and PDB:3OQ9 in yellow). In addition to the displacement of its juxtamembrane region, note the transformation of the α helices 5 and 6 within the death domain into a long stem helix. (B) Superimposition of Holo and Apo structures of the CD95 death domain (PDB:3OQ9 in yellow and PDB:1DDF in red). Note that there is still a conformational rearrangement of helices 5 and 6, but with a limited amplitude. (C) Different X-ray structures of ICD and their orientation toward the plasma membrane. Panels α to δ: proposed orientations of the tetrameric crystal structure of CD95:FADD complex (only CD95 is depicted). The N-terminal region of the death domain starting at N223 is the closest residue to TM and is labeled with black spheres. Chains in red seem correctly oriented regarding the plasma membrane, but the orientation of chains in yellow renders the position of the tetramer improbable. Panels γ to δ: only the closest dimers to the membrane are considered. Drawing in γ represents the most probable orientation toward the membrane. (D) Orientation of the pentameric CD95-DD, taking as reference the protomer showed in Figure 2B. Note that using this model, one DD is inserted into the plasma membrane. β. Optimized orientation of the pentameric CD95-DD regarding the position of the amino terminal residues K231 and Y232 (black balls and sticks) to the plasma membrane. Note the asymmetry of the structure, particularly for chain (A) (arrow).