Figure 3. Purification and Mass Spectrometry of Golgi Outposts.
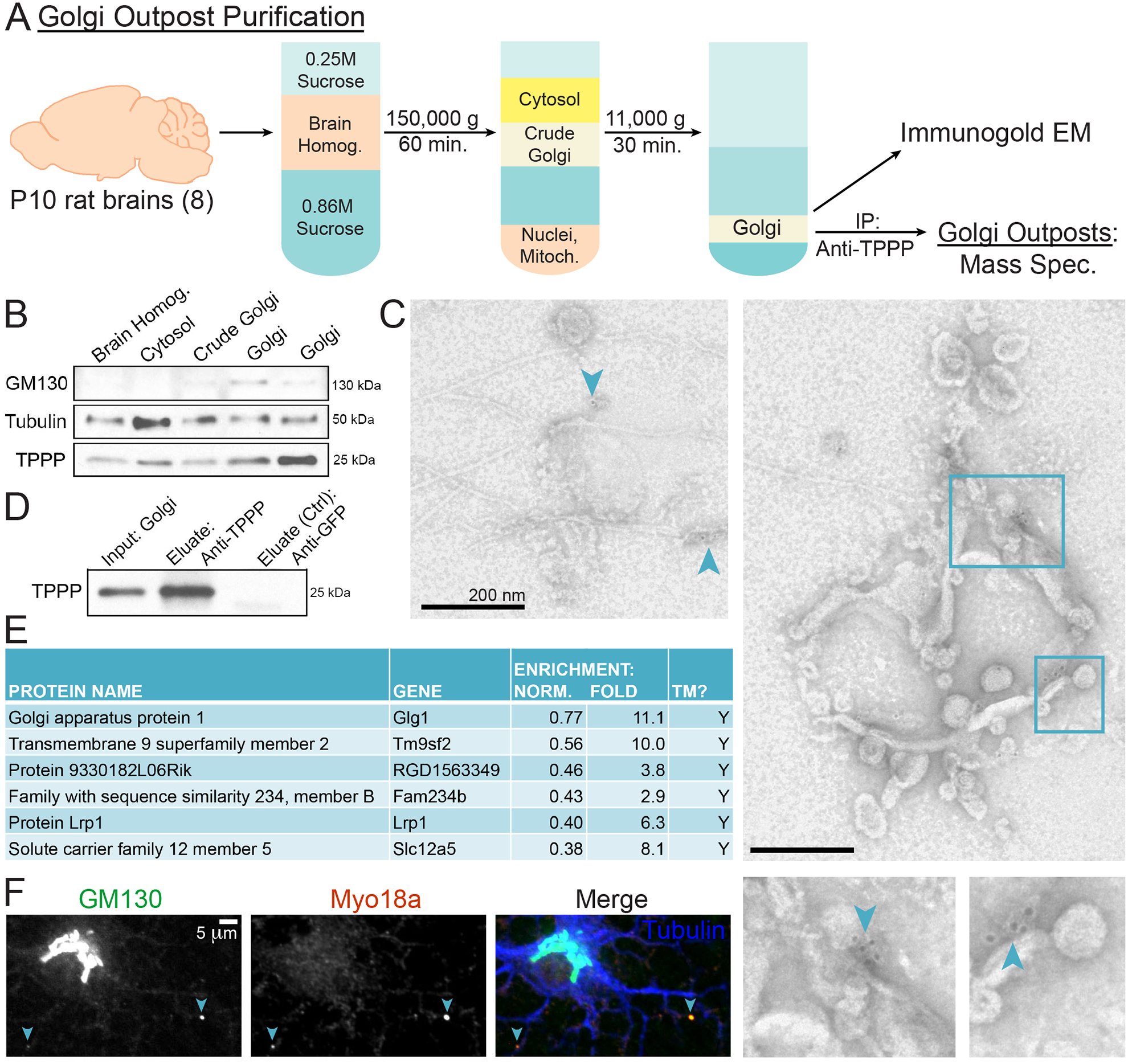
(A) Schematic of Golgi outpost purification from P10 rat brains using differential sucrose-gradient ultracentrifugation. Resulting purified Golgi was then subject to: 1) immuno-EM and 2) IP against TPPP in order to isolate Golgi outposts for MS.
(B) Western blots of Golgi purification fractions show enrichment of GM130 and TPPP. 2 replicates of purified Golgi fractions are shown.
(C) Immuno-EM of purified Golgi that associate with anti-TPPP gold beads. Images show multi-vesicular structures with microtubules emanating from them. Arrowheads point to anti-TPPP gold beads. Bounding boxes are enlarged.
(D) Western blot of anti-TPPP IP using purified Golgi as the input. The negative control used an anti-GFP antibody.
(E) MS of TPPP-associated Golgi outpost proteins. Proteins were sorted by a normalized enrichment value with 1 assigned to the protein with the highest number of spectral counts in each trial. “TM” indicates a protein with transmembrane domain(s) predicted by UniProt. n = 3 biological replicates.
(F) Confocal micrograph of a rat DIV4 oligodendrocyte immunostained against GM130, MYO18A, and tubulin.
See also Figure S3 and Table 1.
