Highlights
-
•
Reconstitution of gums from arabinogalactan proteins (AGP) of Acacia gum.
-
•
Experimental design to optimize their emulsions properties.
-
•
Relevant adsorption of high molar protein-rich AGPs at the interface.
-
•
Combined effect of high molar protein-rich AGPS and total concentration.
-
•
Role of apparent viscosity of bulk to long-term stability.
Keywords: Acacia gum, Limonene, high molar mass protein-rich AGPs, Viscosity, Emulsifying stabilizing properties
Abstract
The impact of high molar mass protein-rich arabinogalactan-proteins (AGPs) on emulsifying properties of Acacia senegal gums were studied using reconstituted gums obtained with two distinct fractions: one containing these specific high molar mass AGPs and the other protein-poor low molar mass AGPs. To produce and stabilize limonene emulsions, the experimental design emphasized not only the role of high molar mass protein-rich AGPs, but also the importance of high total concentration. At low protein contents, reconstituted gums required a slightly higher content in high molar mass protein-rich AGPs than original A. senegal gum, that confirmed the role of low molar mass protein-rich AGPs in the adsorption at interfaces. The comparison of the creaming index between original and reconstituted gums as well as the monitoring of instability phenomena by turbiscan up to 30 days clearly demonstrated the prevalent impact of the bulk apparent viscosity in the long-term stability of emulsions.
1. Introduction
Acacia gum is a natural emulsifier obtained from the trunk and branches of Acacia trees (Sanchez et al., 2018). Two species of gums, A. senegal and A. seyal, are authorized by FAO (1999) and commonly used (Arabic Gums, E414 EC) to stabilize oil in water emulsions, in particular aroma compounds in beverages. Both gums are composed by a continuum of arabinogalactan proteins (AGPs) precipitating in the presence of Yariv’s reagent (Osman et al., 1995). They were chiefly characterized by different molar masses (between 2.8 × 105 and 2.5 × 106 g.mol−1) and protein content (Islam et al., 1997, Mejia Tamayo et al., 2018, Randall et al., 1989, Renard et al., 2006). The latter is about 3% and 1% for A. senegal and A. seyal, respectively. The interfacial activity of AGP has been attributed to the presence of this proteinaceous moiety and to specific high molar mass AGP fractions (Al-Assaf et al., 2006, Randall et al., 1989). The emulsion stabilization, on the other hand, is mainly attributed to the charged and hydrated carbohydrate portion, contributing to viscosity, steric, and electrostatic effects (Chanamai & McClements, 2001). Such a description is consistent with the ‘wattle blossom model’ used to describe AGPs (Fincher, Stone, & Clarke, 1983), where apolar protein residues anchor at the oil-in-water interface and the protruding hydrophilic and charged carbohydrate blocks attached to proteinaceous chain provide a strong electrosteric barrier against flocculation and coalescence (Islam et al., 1997, Dickinson et al., 1988, Bai et al., 2016). A. senegal is generally more efficient to form more stable liquid emulsions than A. seyal. This can be mostly related to the difference in the protein content and structure (e.g. molar mass distribution and protein accessibility to the solvent) of AGPs.
The separation of A. senegal gums on hydrophobic interaction chromatography (HIC), resulted in three main fractions historically named arabinogalactan-peptide (AG or HIC-F1), arabinogalactan-protein (AGP or HIC-F2) and glycoprotein (GP or HIC-F3) which differ by their mean molar mass (Mw), their high Mw AGPs content (AGPs with Mw upper than 106 g.mol−1), and protein content (between 0.4 and 13%) (Randall et al., 1989, Renard et al., 2006).
The efficiency of different gum fractions of A. senegal obtained by size exclusion chromatography (SEC) and by HIC to stabilize citrus oil emulsions was compared taking into account the nitrogen and protein content (Ray, Bird, Iacobucci, & Clark, 1995). The emulsions produced using the same nitrogen level with the whole gum (2.2% protein content) or a SEC fraction (mean Mw of 6.5 × 106 g.mol−1 and 6% protein content) were characterized by a similar droplet size. The use of another fraction with low mean molar mass of 3.4 × 105 g.mol−1 and 2.1% protein content induced lower emulsion droplet size than whole gum but with high instability of emulsion. This study has demonstrated that for comparable protein content, the fractions can produce either good or poor emulsions depending on their molar mass. In addition, it was also reported by the same authors that a more stable emulsion can be made using a mixture of protein-rich low and high molar mass fractions than using either fraction alone.
These previous experiments demonstrated that the protein content alone was not enough to explain the emulsion efficiency of A. senegal gum, and it also proved that the molar mass distribution of protein-rich AGPs seemed to play an important role. This contribution was later tentatively studied using, (i) increased concentrations of gum, or naturally variable gums generating various amounts of AGPs for a same concentration (Bai et al., 2017, Chanamai and McClements, 2001, Nakauma et al., 2008) (ii) modified gums through controlled Maillard reactions having increased amounts of aggregated AGP (Al-Assaf et al., 2007, Aoki et al., 2007, Castellani et al., 2010, Xiang et al., 2015, Han et al., 2019), or (iii) AGP-rich fractions of gums isolated by HIC chromatographic method (Fauconnier et al., 2000, Ray et al., 1995). It was also shown by gel permeation chromatography (GPC) on supernatants after removing oil droplets that protein-rich fractions adsorb strongly at the oil-in-water interfaces (Flindt et al., 2005, Randall et al., 1988). However, while a preferential adsorption of high molar mass protein-rich AGP fractions occurred, all molecular fractions seemed to be present at interfaces having different adsorption kinetics (Flindt et al., 2005, Padala et al., 2009, Alftren et al., 2012, Atgié et al., 2019).
There is no doubt about the important surface activity of protein-rich AGP fractions and of the important role of high Mw fractions in the stabilization of emulsions. However, in almost all works, Acacia gums from different origins and stage of maturation (batches) were used as well as different methodological approaches and experimental conditions, that renders the analysis of the specific impact of protein-rich AGP macromolecules on emulsion properties difficult.
The present study aimed to highlight the role of high molar mass protein-rich AGPs content on emulsion formation and stability. In our approach and in order to overcome the natural variability of gums from different batches, reconstituted A. senegal gums with controlled high molar mass protein-rich AGPs concentrations were formulated by mixing two selected fractions of A. senegal gum originating from the same gum’s batch. One of the fraction (named HIC-F1) was mainly composed of protein-poor low molar mass AGPs and the other (named IEC-F1) contained exclusively high molar mass protein-rich AGPs. This latter was separated by ion exchange chromatography (IEC), through an adapted procedure, developed by our team (Apolinar-Valiente et al., 2019) and based on the work of Osman et al. (1995) about the fractionation of the AGP-aggregate rich Acacia gums. An experimental design was used to optimize the formulation of a “model” reconstituted gum allowing long term stability of limonene emulsions. This allowed as well a controlled modulation of the rate of high molar mass protein-rich AGPs and thus final emulsifying properties of gums. Comparisons between reconstituted gums and the original Acacia senegal gum were conducted in order to better delineate the global effects of AGPs including concentration and viscosity from the specific effect of high molar mass protein-rich AGPs.
2. Materials and methods
2.1. Materials
Acacia senegal (A. senegal, batch n° OF152413) gum powder was provided by ALLAND & ROBERT Company-Natural and organic gums (Port mort, France). The biochemical composition was previously characterized showing a 2.2 wt% protein content (Lopez-Torrez et al., 2015, Mejia Tamayo et al., 2018).
The reconstituted gums were formulated by mixing at different rates two selected AGP fractions obtained from the original A. senegal gum (batch n° OF152413). The first fraction, called HIC-F1, was obtained according to the classical fractionation method via Hydrophobic Interaction Chromatography (HIC) (Randall et al., 1989, Renard et al., 2006) and the second one, named IEC-F1, was obtained by a recently used Ion Exchange Chromatography (IEC) approach (Apolinar-Valiente et al., 2019). HIC was performed at room temperature on a Phenyl-Sepharose CL-4B (Pharmacia, Uppsala, Sweden) column (75 × 2.6 cm) equilibrated with degassed 4.2 M NaCl. Acacia gum (10 wt%) in 4.2 M NaCl was loaded and eluted successively by 4.2 M NaCl (HIC-F1 fraction), 2 M NaCl (HIC-F2 fraction), and finally water (HIC-F3 fraction) at a flow rate of 46.2 mL.h−1. IEC separation was performed at room temperature on DEAE Sephacel (Sigma Aldrich, St. Louis, Mo) column (54 × 20 cm). The column was firstly equilibrated with degassed water. Dissolved A. senegal gum was loaded and eluted by water (around 10 L) at a flow rate of 40 mL min−1 to obtain a first fraction called IEC-F1. The appropriate fractions were pooled, extensively dialyzed against distilled water and spray-dried.
HIC-F1 showed a small protein content (0.49% of protein) and is mainly composed by low molar mass AGPs while IEC-F1 contains only high molar mass protein-rich AGPs (9.4 wt% of protein) (Mejia-Tamayo, 2018, Apolinar-Valiente et al., 2019). The rate of high molar mass protein-rich AGPs (Mw > 7.5 × 105 g.mol−1), experimentally determined by size exclusion chromatography coupled to multi-angle laser light scattering (SEC-MALS), corresponded to 7%, 100% and 14% for HIC-F1, IEC-F1 and initial A. senegal, respectively. The percentage of proteins for each concentration of the reconstituted gum was calculated taking into account the percentage of each fraction in reconstituted gum and their respective protein content. As an example, a reconstituted gum with a total gum concentration of 4% and containing 5% of IEC-F1 was characterized by a protein content of 0.0374% calculated from Eq. (1). It corresponds also to a high Mw protein-rich AGPs content of 0.466%.
| (1) |
The detailed biochemical composition of the two fractions (8% moisture) and A. senegal gum (12% moisture) are presented in Supplementary Table I.1.
Acetate buffer (10 mM, pH 5) was prepared with anhydrous glacial acetic acid and sodium acetate trihydrate powder provided by Merck KGaA (Darmstadt, Germany) and Fluka-Sigma Aldrich (Saint-Quentin Fallavier, France), respectively.
Limonene (97% of purity) used as oil phase for emulsion was purchased from Fluka-Sigma Aldrich (Saint-Quentin Fallavier, France).
2.2. Preparation of the dispersions of Acacia gum and the mixture of fractions
The initial Acacia gum and purified fractions were dispersed in 10 mM acetate buffer (pH 5) and stirred during 8 h at room temperature to ensure biopolymer hydration. The total concentrations of A. senegal gum and reconstituted gums were based on wet weight of powder and varied between 0.5 and 20 wt%. The reconstituted A. senegal gums were formulated according to an experimental design and resulted from the mixing of HIC-F1 and IEC-F1 fractions. Thus, the total concentration varied between 1.3 and 19.7 wt% with IEC-F1 content ranging from 0.9 to 29.1 wt% of the total concentration (Table 1). Before use, all gum dispersions were centrifuged at 5000 rpm and 25 °C during 20 min to eliminate insoluble matters.
Table 1.
Matrix of the central composite design of two variables in units along with the experimental response and the validation experiments.
| Treatment run | IEC-F1 content (%) | Total concentration of gum fractions mixture (%) | Apparent viscosity (mPa.s) | Response variable | ||
|---|---|---|---|---|---|---|
| D4,3 (µm) | Delay time (min) | CI at 24 h (%) | ||||
| 1 | 5 | 4 | 4.1 | 1.19 | 80 | 5.42 |
| 2 | 5 | 17 | 48.2 | 0.66 | 327 | 3.41 |
| 3 | 25 | 4 | 4.4 | 0.82 | 95 | 6.18 |
| 4 | 25 | 17 | 81.2 | 0.61 | 654 | 2.92 |
| 5(C) | 15 | 10.5 | 19.6 | 0.67 | 241 | 4.06 |
| 6(C) | 15 | 10.5 | 22.5 | 0.68 | 275 | 3.63 |
| 7* | 0.9 | 10.5 | 16.1 | 1.08 | 176 | 4.51 |
| 8* | 29.1 | 10.5 | 28.1 | 0.63 | 355 | 3.04 |
| 9* | 15 | 1.3 | 1.9 | 1.43 | 18 | 7.52 |
| 10 | 15 | 19.7 | nd | 0.59 | 584 | 3.66 |
| 11(C) | 15 | 10.5 | 19.2 | 0.66 | 247 | 4.55 |
| 12(C) | 15 | 10.5 | 20.7 | 0.68 | 230 | 4.58 |
| 13 exp | 15 | 4 | 4.6 | 0.88 | 88 | 6.40 |
| 13 pred | 1.03 | 75 | 6.18 | |||
| 14 exp | 5 | 10.5 | 14.3 | 0.78 | 174 | 4.99 |
| 14 pred | 0.90 | 183 | 4.17 | |||
| 15 exp | 0.858 | 17 | 26.9 | 0.78 | 262 | 4.42 |
| 15 pred | 0.77 | 281 | 3.73 | |||
| 16 exp | 0.858 | 4 | 3.6 | 1.62 | 38 | 8.00 |
| 16 pred | 1.63 | 47 | 6.87 | |||
C, center point; *, star point (axial). The values of D4,3 in bold were excluded from the worksheet during data treatment. nd: non determined. Exp: experimental values, Pred: Predicted values
2.3. Methods
2.3.1. Apparent viscosity of Acacia gum dispersions
The apparent viscosity of the aqueous phase of Acacia gum dispersions before emulsion preparation was measured at 25 °C using a rotating stress-controlled rheometer (RheoCompass MCR 702, Anton Paar, Les Ulis, France) equipped with a sanded cylindrical geometry (cup diameter: 22 mm; bob diameter 19.997 mm). Rheological measurements could not be performed on emulsions because of their instability in several experimental conditions. The apparent viscosity of samples was measured at increasing shear rates from 0.1 to 1000 s−1. For all dispersions, the flow curves showed a Newtonian behavior with a plateau at high shear rate (data not shown). The apparent viscosity obtained at a shear rate of 100 s−1 was used to compare samples.
2.3.2. Preparation of limonene emulsions
The oil in water emulsions were prepared by adding 5 wt% of limonene to 95 wt% of Acacia gum dispersions (original or reconstituted) without addition of weighting agent. Coarse emulsions were first prepared using rotor/stator homogenizer (Silverson L4RT, Evry, France) equipped with a square hole high shear screen stator at 7500 rpm for 5 min at room temperature (~25 °C). Coarse emulsions were homogenized using a microfluidizer with a F12Y diamond interaction chamber (LM20, Microfluidics Corporation, MA, USA) using a pressure of 440 bars and 1 pass. The outlet microfluidizer coil was immersed in a water bath to maintain temperature at around 25 °C.
2.3.3. Emulsion droplet size measurements
The mean diameter and diameter distribution of emulsion droplets were determined by laser light scattering using a Mastersizer 2000 (Malvern Instrument, Orsay, France). Refractive index of 1.33 for water and 1.47 for limonene were used. For all emulsions, three cycles of measurements were performed 10 min after the emulsification step, using an obscuration value of around 10%. The mean droplet diameter was expressed as the volume mean diameter D4,3 and the polydispersity index for each sample was calculated.
2.3.4. Emulsion stability measurements
The colloidal stability of emulsions was monitored using a vertical scan light scattering analyzer type Turbiscan Tower (Formulaction, Toulouse, France) equipped with a pulsed near infrared light source (λ = 880 nm) and two synchronous transmission (T) and backscattering (BS) detectors. The Turbiscan allows to monitor the emulsion stability in time and space and to characterize the emulsion instability phenomena such as creaming, sedimentation and coalescence. The backscattering (BS in %) and transmittance (T in %) signals were recorded and analyzed using the TowerSoft software version 1.10.36. 15 mL emulsion sample (equivalent to ~3.5 cm height) were loaded into cylindrical glass tubes 15 min after emulsion preparation and scanned throughout its entire height. BS and T were recorded every 110 s during the first 24 h and then once a day during one month of storage at 25 °C.
During storage, emulsions generally separated into a top opaque layer (creaming), a turbid layer in the middle (emulsion) and a bottom transparent layer (clarification). In order to compare the stability of samples, the differences in backscattering (BS) and transmission (T) profiles between scans recorded at different times and the first recorded scan were plotted (ΔBS and ΔT, in %). Based on the evolution of these signals during storage, the delay time, the creaming index and the turbiscan stability index (TSI) were calculated and used to compare the colloidal stability of all produced emulsions. The delay time corresponds to the time for which the backscattering of emulsions at the bottom zone (clarification peak) decreased from 10% of its height. The creaming index (CI) was usually calculated after 24 h of storage from Hs, the serum top (turbid and transparent) layer and Ht, the total height of emulsion (Gu, Decker, & McClements, 2005) (Eq. (2)).
| 2 |
Due to the strong instability of the emulsions, Hs was chosen to be, in our case, the height of the lower serum layer (clarification) determined at the apex of transmittance at the bottom of sample. This allowed a better discrimination among emulsions prepared with limonene.
The Turbiscan stability index (TSI) corresponded to the signal variation at definite positions (h) throughout various height (H) ranges of the sample between the scani and the scani−1. TSI calculated over the entire tube height takes into account the ensemble of destabilization phenomena occurring during storage (creaming, flocculation/coalescence, clarification). It is determined according to Eq. (3).
| 3 |
2.3.5. Experimental design
Response surface methodology was used to evaluate the effect of two independent variables on the droplet size, the delay time and creaming index of emulsions. The first independent variable was the amount of IEC-F1 (, 0.9–29.1% of relative total concentration), and the second one was the total concentration of the A. senegal reconstituted gums (, 1.3–19.7 wt%), resulting from the mixing of fractions HIC-F1 and IEC-F1. The experiments were planned using central composite design (CCD) according to a 22 factorial plan with star and central points. In Table 1, the coded and uncoded independent variables were listed. Eight experimental settings and four central points were carried out randomly. The repeatability of the emulsification method was estimated by repeating the center points (4 times). The experimental design and data analysis were generated using Modde 12 (Sartorius Stedim Biotech, Sweden). For each variable, a second-order polynomial equation was constructed to estimate the responses and optimize the parameter (Eq. (4)):
| 4 |
where y is the estimated dependent variable, a constant, the linear effect coefficient, the quadratic effect coefficient, the interaction coefficient for the regression equation, and the independent variables. The adequacy of the models was evaluated by the lack of fit, coefficient of determination (R2) and adjusted R2 (Adj-R2) analysis.
2.3.6. Statistical analysis
The repeatability of the experimental design was assessed on the 4 replications of the central points and the standard deviations were 1.2% for D4,3, 7.7% for the delay time and 10.7%, for CI. The mean droplet diameter was expressed as the volume mean diameter D4,3 of three cycles of measurements. For Turbiscan measurements, each sample was analyzed in duplicate. Rheological measurements of apparent viscosity were done once on each sample due to the high amount of sample needed, but the repeatability was also assessed on the 4 replications of central points of experimental design as previously indicated. As for emulsions made with initial gum, 3 independent emulsions were prepared for analyses.
3. Results
We were interested in the present study to have a more complete look on the role of high Mw protein-rich AGPs in emulsifying properties of A. senegal gum. We chose to formulate well controlled reconstituted gums formed by mixing two purified fractions (IEC-F1 and HIC-F1) obtained from the same batch of Acacia senegal gum. By varying the proportion and the amount of the two fractions, it was possible to modify the high molar mass protein-rich AGPs content of the reconstituted gums.
3.1. ANOVA study of the experimental design
As the amount of purified fractions was limited, a response surface methodology was used to study the effect of IEC-F1 content (0.9–29.1% of total concentration) and total concentration (IEC-F1 and HIC-F1 mixture varying between 1.3 and 19.7 wt%) on the formation and stability of limonene-in-water emulsions. The studied variables were the volumetric droplet diameter (D4,3), the delay time, and creaming index (CI). The two latter describe the stability during the first 24 h after the formation of emulsions. Indeed, the emulsions were quickly unstable as observed on the Turbiscan backscattering profiles (Supplementary data Fig. 1.1), highlighting the fast emergence and expansion of multiple destabilization phenomena such as creaming, coalescence/flocculation and clarification which took place in all emulsions at different levels depending on the total concentration and the proportion of each fraction in the mixture (Supplementary data Fig. 1.1).
The randomized runs of the experiments (1–12) and experimental responses are presented in Table 1. The second-order polynomial response surface model was carried out on the values of each measured variable. Analysis of variance (ANOVA) was used in order to determine the statistical significance of regression coefficients and for the fitting of the model. Table 2 showed the estimated regression coefficients of the model for the response variables and the corresponding coefficients of determination (R2) and adj-R2. A significant lack of fit (p-value < 0.05) indicates the failure of the model representing the experimental data and the response predictor is discarded (Koocheki, Taherian, Razavi, & Bostan, 2009). If the response surface model fitted well for the response variable, it would indicate that the variable was assessed as a function of linear, quadratic and interactions effects of IEC-F1 content and total concentration.
Table 2.
Table of ANOVA for the experimental variables as a linear, quadratic and interaction terms of each response variable and corresponding coefficients for the predictive models.
| Source | D4,3 (µm) | Delay time (min) | CI (%) | |||
|---|---|---|---|---|---|---|
| Coefficient | p-value | Coefficient | p-value | Coefficient | p-value | |
| Lack of fit | 0.114 | 0.514 | 0.427 | |||
| 0.997 | 0.995 | 0.932 | ||||
| Adj- | 0.994 | 0.990 | 0.874 | |||
| Linear | ||||||
| −0.160 | <0.0001* | 74.399 | <0.0001* | −0.227 | 0.2291 | |
| −0.237 | <0.0001* | 200.821 | <0.0001* | −1.340 | 0.0002* | |
| Quadratic | ||||||
| 0.088 | <0.0001* | 10.063 | 0.228 | −0.2665 | 0.2102 | |
| 0.123 | 0.00014* | 27.818 | 0.0099* | 0.64101 | 0.0149* | |
| Interaction | ||||||
| 0.137 | 0.00014* | 78 | 0.00017* | −0.309 | 0.24541 | |
: the estimated linear coefficient of the quadratic polynomial equations. : the estimated quadratic coefficient of the quadratic polynomial equations : the estimated interactive coefficient of the quadratic polynomial equations. (1): IEC-F1 content; (2): total concentration of gum. * stands for a significant value.
We observed that the particle size distribution was monomodal for all the samples indicating that total gum concentration and the process conditions were sufficient to form small uniform droplets (see Supplementary data Fig. 1.2).
For the D4,3 parameter, two values (highlighted in bold in Table 1) were excluded from the worksheet in order to obtain a reduced fit model. These values were the highest and were obtained for low total concentration and low AGP content. It can be expected that the variability of D4,3 was higher when large droplets were formed leading to unstable emulsions. For the CI and delay time variables, all experimental values were used to build the regression models. A high coefficient of determination (R2) and adjusted R2 (adj-R2), and a non-significant lack of fit (p-value > 0.05), were observed for all variables indicating that the proposed models are adapted to fit the experimental values (Table 2). (Predicted and experimental values for each variable are shown in Supplementary data Fig. 1.3).
Regarding the regression coefficients of the reduced model of D4,3 (Table 2), significant p-values were found for linear and quadratic effect of IEC-F1 concentration and total gum concentration and for interaction effects. For the delay time, the significant p-values were only found for linear and quadratic effect of total gum concentration and for interaction effects. The ANOVA study of regression coefficients of CI indicated that linear and quadratic effects of total concentration were significant and that the major effect was induced by the linear effect. This highlighted the importance of total concentration for delay time and CI, then on parameters characterizing the stability of emulsions in the short term. However, the former was also affected by IEC-F1 content through the interaction effects.
3.2. Validation of the model and optimization of the composition of reconstituted gum
For validation of the correlation, new experiments (13–16) were carried out, and the experimental variables, i.e. the D4,3, the creaming index (CI) and the delay time were compared with predicted values from the regression equation. The results were reported in Table 1.
The predicted values for all variables were close to the experimental values demonstrating that the obtained model was relevant. The determination coefficients were equally high for D4,3 (R2 = 0.96), delay time (R2 = 0.99) and creaming index (R2 = 0.94). Finally, the optimized formulation conditions, for moderated process conditions (440 bars, 1 pass) with limonene, determined through the model was 17.5% total gum concentration containing 24.1% of IEC-F1 (corresponding to 4.21% of total IEC-F1 concentration). For this specific reconstituted gum, the theoretical models predicted values for D4,3 of 0.62 µm, delay time of 649 min and creaming index of 2.77%. The relative high total concentration and IEC-F1 content would probably diminish for stronger process conditions and/or using another dispersed phase (Zhang, Peppard, & Reineccius, 2015).
3.3. Effect of high Mw protein-rich AGPs on emulsion droplet size, delay time and creaming index
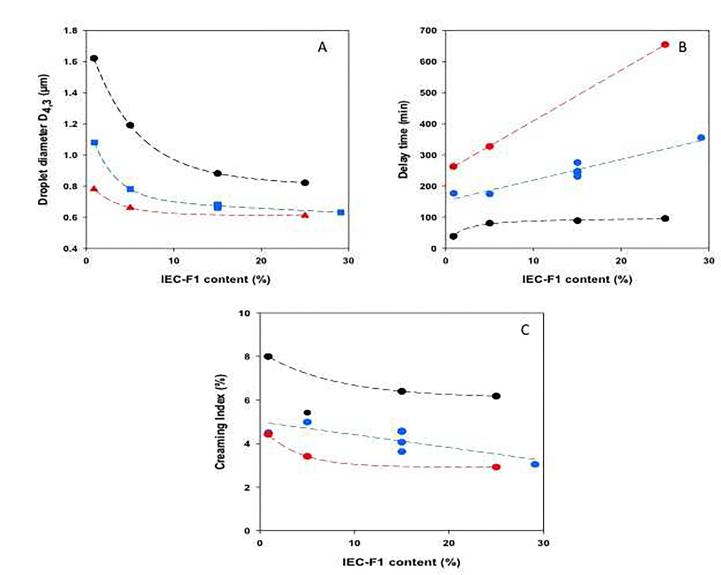
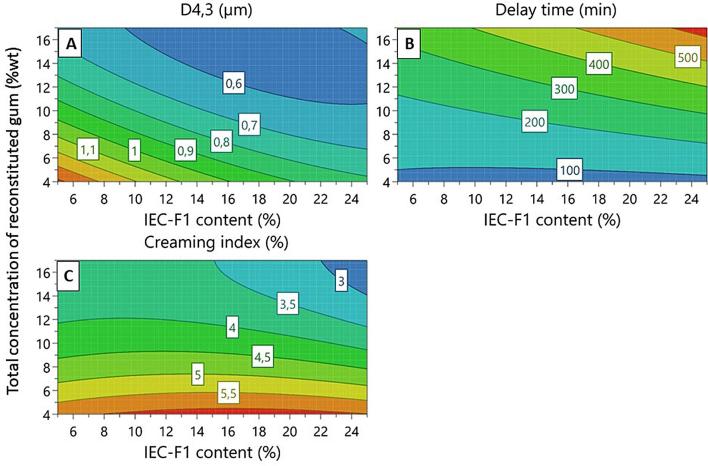
The relationship between the relative IEC-F1 content and total AGP concentration in mixtures, and the different emulsion stability variables (D4,3, delay time and CI) were represented using the easiest optimization technique called “one variable at a time” (Fig. 1) and the surface response (Fig. 2).
Fig. 1.
Fig. 2.
Fig. 1A shows the effect of IEC-F1 content on the volumetric emulsion droplet diameter D4,3, at three total AGP concentrations (4, 10.5 and 17 wt%). As expected, an increase of the relative high Mw protein-rich AGP content induced a decrease of D4,3 for the three total concentrations studied. However, the specific effect of these AGPs was clearly related to the total AGP concentrations. It was especially significant in emulsions prepared at the lowest total gum concentration, i.e. 4 wt%. At 17% total gum concentration, a specific effect of high Mw protein-rich AGPs is much less obvious. When the IEC-F1 content raised from 0.9 to 25%, the reduction of droplet size was around 49% for a total concentration of 4 wt% compared to 22% at 17 wt%. It can be supposed that above around 20–25% of high molar Mw protein-rich AGPs, the relative content in these AGPs should not be the critical parameter. These results suggested the combined effect between high Mw protein-rich AGPs and total gum concentration on droplet diameter of limonene emulsions. This is also clearly evidenced from surface response graphs where a reduction of total concentration from 16% to 10% needed a compensation of around 16% of added IEC-F1 content to obtain the same droplet size (Fig. 2A). In turn, this shows that both high molar mass and low molar mass protein-rich AGPs are important in defining surface properties of Acacia gum. Accordingly, it was described that different protein rich AGPs displaying a broad range of molar masses specifically adsorbed at the oil-in-water interface (Randall et al., 1988, Alftren et al., 2012, Atgié et al., 2019).
Regarding the emulsion stability, the delay time increased with total concentration and IEC-F1 content. Contrary to D4,3, the IEC-F1 effect was more pronounced on delay time at the highest total concentrations (Figs. 1B and 2B). As shown in Fig. 2B, to reach a delay time of 300 min for a total concentration of 12 wt%, it needed to add 18% of IEC-F1 while a weakest IEC-F1 amount (6%) was sufficient at 16% of total concentration. On the other hand, the creaming index (CI) slightly varied with the IEC-F1 content for a given total concentration (Figs. 1C and 2C). In this case, the increase of total concentration was the prevalent factor for the decrease of CI. These results suggested that a critical high Mw protein-rich AGP concentration must exist below which poor emulsion stability has to be expected.
Emulsifying properties of Acacia gum are related not only to the specific presence of high Mw protein-rich AGPs (Dickinson et al., 1988, Ray et al., 1995), (then of rather large dimension macromolecules), but also to the global richness in protein of gums (Anderson and Weiping, 1991, Dickinson et al., 1988, Ray et al., 1995). The impact of protein concentration on the emulsifying properties of gums is summarized in Fig. 3.
Fig. 3.
D4,3 markedly decreased up to a protein content of about 0.2 wt% for both emulsions made with reconstituted or original gums (Fig. 3A), with a concomitant increase in droplet size distribution expressed by the polydispersity index which varied between 0.632 and 1.178 (Fig. 3B). No further decrease of D4,3 was observed above this 0.2 wt% value, and that can be viewed as a gum-dependent critical protein concentration. It corresponded to about 2 wt% of high Mw AGPs. Below this critical concentration, the droplet diameter was smaller for emulsions made from original A. senegal gums (Fig. 3A and inset). This difference was small but significant. Interestingly for the same low protein concentrations, delay time values (Fig. 3C) were slightly weaker for emulsions made from original compared to reconstituted gums. Delay time difference was more pronounced above the critical 0.2 wt% protein content emphasizing the positive influence of high Mw protein-rich AGPs on emulsion stability. By contrast, the creaming index showed a stabilizing effect of reconstituted gums below 0.2 wt% critical concentration as compared to original A. senegal (Fig. 3D). For higher protein concentration, the emulsion stability within 24 h was not different between the two used gums.
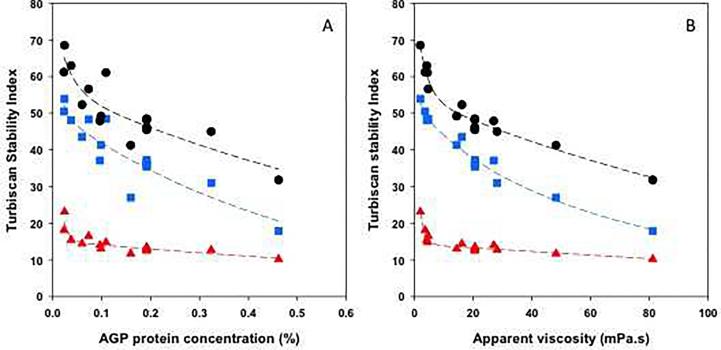
The stability of emulsions within >1-month storage was also considered by calculating the Turbiscan stability index (TSI). This parameter includes the ensemble of emulsion stability phenomena (clarification, creaming and flocculation/coalescence). In Fig. 4A, only the global TSI calculated at 1, 7 and 30 days for emulsions prepared with reconstituted gums are plotted according to the AGP protein concentration. Indeed, similar results were gathered at 46 days compared to 30 days. The stability of emulsions obviously decreased with the storage duration but destabilization was impaired as the AGP protein concentration increased. A strong variation of TSI was observed for emulsions prepared with a similar AGP protein concentration (Fig. 4A). For instance, the emulsions prepared with 0.1087% and 0.0982% of AGP protein concentration showed some huge difference in their TSI values (61.1 against 49.2) after 30 days of storage (Fig. 4A). In the same vein, the emulsion containing 0.159% of AGP protein concentration was more stable than that prepared with 0.324% of AGP protein concentration after 30 days of storage (TSI values of 41.2 and 45, respectively) (Fig. 4A). The most stable emulsion was characterized by highest bulk apparent viscosity (48.2 mPa s against 28.1 mPa s). These results highlighted that the AGP protein concentration was not sufficient to explain the emulsion colloidal stability during a long storage period. More interestingly, a coherent emulsion stability profiles could be obtained when plotting TSI not as a function of AGP protein concentration but as a function of the apparent viscosity of starting reconstituted gums dispersions (Fig. 4B). The effect of AGP dispersion apparent viscosity has scarcely been studied when looking at their emulsifying efficiency. However, it is certainly an important parameter that needs to be taken into consideration to delineate specific interfacial effects of high Mw AGPs from their hydrodynamic effects on aqueous solvent properties.
Fig. 4.
4. Discussion
The specific effect of high Mw protein-rich arabinogalactan-protein (AGP) and total Acacia gum concentration on the formation and stability of limonene-based emulsions was studied using an experimental design. The focus was put on the ability of these specific AGPs to adsorb and stabilize oil-in-water interfaces. These emulsifying properties were described by the emulsion droplet volumetric diameter (D4,3), the delay time (time to reach 10% emulsion stability change at the bottom of the sample), the creaming index (CI) that showed instability after 24 h of storage, and the TSI that informed on the whole instability of the emulsions. Moreover, the behaviour of reconstituted gums was compared to original gum.
In order to tentatively explain the results obtained, we first need to be clear on the different AGP characteristics of the two gums considered. The initial Acacia gum contained three families of AGP when analyzed using hydrophobic interaction chromatography, HIC-F1, HIC-F2 and HIC-F3 (Renard et al., 2006, Mejia Tamayo et al., 2018). The first one is largely in majority (~85%) but poor in proteins. The surface properties are mainly due to the protein rich ones, HIC-F2 that contains only large AGPs (Mw ~ 24 × 105 g.mol−1) but especially HIC-F3 that contains both small (Mw ~ 3 × 105 g.mol−1) and high Mw AGPs (Mw > 7.5 × 105 g.mol−1) (Renard et al., 2006, Castellani et al., 2010).
Reconstituted Acacia gums were based on mixing two fractions purified from initial Acacia gum, namely the HIC-F1 fraction and IEC-F1, that was obtained by ion exchange chromatography (Supplementary data Table I1). The IEC-F1 fraction contained only high Mw protein-rich AGPs (Mw: 3.0 × 106 g.mol−1) with an intrinsic viscosity value [η] that was twice that of starting gum (Supplementary data Table I2). Chemical analysis of amino-acids composing the IEC-F1 protein moiety indicated the IEC-F1 fraction was composed by large molar mass HIC-F2 and HIC-F3 AGPs mixture (Apolinar-Valiente et al., 2019). Its adiabatic compressibility (−9.4 × 10−11 Pa−1) was consequently intermediate between those of HIC-F2 (−14.4 × 10-11 Pa−1) and HIC-F3 (−1 × 10-11 Pa−1) (Mejia-Tamayo, 2018). Adiabatic compressibility can be related both to a weak polarity (Mejia Tamayo et al., 2018, Apolinar-Valiente et al., 2019) and a high surface activity (Razumovsky & Damodaran, 1999) of these protein-rich AGPs. Increasing the amount of IEC-F1 in mixtures with HIC-F1 then mostly increased the content in high Mw protein-rich AGPs and potentially improved its emulsifying ability. Indeed, a positive correlation between the adiabatic compressibility and the interfacial properties of food proteins was previously shown (Gekko & Yamagami, 1991).
A critical high Mw protein-rich AGP concentration of about 2 wt% was identified below or above where different changes in emulsion structure and stability could be observed. This was equivalent to 0.23 wt% of protein, a critical value also found in other studies dealing with Acacia gum-stabilized emulsions (Huang et al., 2001, Randall et al., 1988). Below about 0.2 wt% protein concentration, emulsions made with reconstituted gums displayed somewhat larger droplets than emulsions made with initial Acacia gum but were clearly more stable against destabilization than the latter (Fig. 3C). Similar result was reported once (Ray et al., 1995). Above this critical protein concentration, the initial droplet diameter D4,3 and limonene emulsion stability were essentially the same using initial Acacia gum or reconstituted gums.
However, the model derived from the experimental design identified that the optimized composition of reconstituted gums was a total concentration of 17.5% containing 24.1% of IEC-F1. It corresponds to 4.21% of high Mw protein-rich AGPs in the final solution. This highlights that if a minimal concentration of 2% is sufficient to the adsorption at oil-interfaces, a higher concentration of 4.21% optimizes emulsions stability as will be further discussed.
The formation of a protective layer at the oil-in-water interface supposes that the surface active molecules, here highly glycosylated glycoproteins, are rapidly transported from water to the created oil-in-water interface in order to decrease interfacial tension. Then the molecules displace interfacial water molecules, interact and rearrange to form a viscoelastic film of macromolecules in equilibrium with those remain unabsorbed (Vogler, 2012). Macromolecular films structurally stabilize emulsions by reducing the rate of droplet recoalescence (Fisher & Parker, 1988). During high shear mixing and high pressure microfluidization, surface active molecules are transported to oil interfaces by diffusion and convection (Dickinson Mauffret, Rolfe, & Woskett, 1989). In classical diffusion-controlled mass transport, small molecules diffuse faster to interfaces than larger ones. On the other hand, in the context of convective mass transport that occurs in turbulent fluids, large protein assemblies are transported faster to interfaces than small proteins (Vogler, 2012). Then small and large molar mass protein-rich AGPs are transported to limonene-in-water interfaces at different rates depending on hydrodynamic conditions. It is then possible that larger droplet diameters observed in emulsions made with reconstituted gums were mainly due to the larger hydrodynamic volume of added AGPs that probably slowed down diffusion-controlled adsorption to interfaces, and it also delayed macromolecular rearrangements, interactions and formation of a thermodynamically stable semi rigid interfacial film. On the contrary, the presence of small Mw protein-rich AGPs in initial gum may favors rapid adsorption and interfacial film formation, leading to smaller emulsion droplet diameter. The larger apparent viscosity of reconstituted gum dispersions at low high Mw protein-rich AGPs content (as an example the viscosity of original Acacia gum having a high Mw protein-rich AGPs content of 0.7% was equal to 2.5 mPa.s against 4.61 mPa.s for a reconstituted gum having a slightly higher rate (0.83%)) (Supplementary data Fig. 1.4) could as well affect the efficiency of the two homogenization stages, then the droplet size, by both an increase of shear stress, that should favor droplet disruption (shear effect), and a reduction of Reynolds number that in turn should decrease the final droplet size (depression of turbulence effects). It is not possible to conclude at this stage without additional experiments using Acacia senegal gum dispersions of constant viscosity and various homogenization conditions.
Limonene emulsions were unstable, presenting multiple destabilization phenomena such as creaming, coalescence/flocculation and clarification. The time needed to detect 10% emulsion stability change (delay time) increased both with gum concentration and the content in high Mw protein-rich AGPs, without reaching a steady value. According to the Stoke’s law, the creaming rate depends on the droplet size but also on the viscosity of the continuous phase (Bai et al., 2017, Zhang et al., 2015, Walstra and Oortwijn, 1982). Since the limonene droplet diameter was almost constant above the critical 0.2 wt% AGP protein concentration (Fig. 3A) and that emulsion delay time did not reach a constant value, it is assumed that the continuous phase viscosity plays an important role in decreasing the extent of emulsion gravitational separation. When the AGP concentration is large, a minor amount of molecules forms an interfacial film while most of them remain dispersed in the continuous phase. The much higher sensitivity of delay time parameter to the content in high Mw AGPs at high gum concentration is also an indication of the prevalent effect in these conditions of continuous phase viscosity (Figs. 1B and 4) that is closely related to the physicochemical properties, as the intrinsic viscosity, of the AGPs dispersed in the continuous phase.
When the concentration of high Mw AGPs was below 0.2 wt%, variation of creaming index showed that emulsions produced with reconstituted gums were more stable than emulsions done with initial gum. It is possible that adsorbed high Mw protein-rich AGPs could form a more elastic interfacial film; then, more stable than that obtained with smaller AGPs (Aoki et al., 2007, Castellani et al., 2010, Xiang et al., 2015). The interfacial rheological film properties of IEC-F1 were recently compared to that of A. senegal gum, and it was observed that a IEC-F1 fraction used at 0.415 wt% AGP concentration was able to form interfacial films with similar rheological characteristics as original A. senegal gum containing 0.7% high Mw protein-rich AGP (Aphibanthammakit, 2018). The role of IEC-F1 in the formation of a strong interfacial film even at very low concentration was clearly demonstrated and justified the strong emulsion stabilizing properties of the reconstituted gums at low total AGP concentration. In addition, the larger hydrodynamic volume of adsorbed AGPs from IEC-F1 may enhance steric repulsions effects that are known to play an important role in the ability of Acacia senegal gum to stabilize emulsions (Dickinson, 2003, Trindade et al., 2008).
Finally, at high Mw protein-rich AGP concentration above 0.2 wt%, the number of surface-active molecules is largely sufficient to rapidly cover the interface and stabilize emulsions so that no difference can be seen between initial and reconstituted gums. The known propensity of protein-rich AGPs to aggregate with the increase in concentration may also smooth the difference in hydrodynamic volume of AGPs from the two used gums (Apolinar-Valiente et al., 2019); thus, contributing to the data similarity obtained for the two kinds of emulsions.
5. Conclusion
In this study, the role of high Mw protein-rich AGPs in emulsifying properties of Acacia gum was investigated by varying their amount in reconstituted gums. The results of the experimental design highlighted the combined effect between high Mw protein-rich AGP content and total gum concentration on the ability to form low droplet size and to stabilize the emulsions. A sufficient content (2%) of high Mw protein-rich AGPs is essential to the formation of the interfacial film at the surface of the oil droplets in the first step of the emulsifying process. Their rich protein content and their enhanced flexibility allow a quick adsorption at the interface. However, the increase of total gum concentration in relation to the increase of bulk apparent density plays a major role in the long-term stability of emulsions studied up to 30 days of storage.
The comparison between reconstituted gum and original gums having equivalent high Mw protein-rich AGPs emphasized the importance of low Mw protein rich AGPs to form emulsion with small droplet size.
From a practical point of view, these results highlighted the importance of how to select gums combining sufficient amount of high Mw protein-rich AGPs and high apparent viscosity to obtain stable emulsion. This could be achieved by increasing the gum concentration to reach these conditions (>20% for Acacia senegal using limonene as dispersed phase), by adding another gum or additive to increase the viscosity. Furthermore, the approach conducted in this study addresses better the interactive roles of the different fractions in term of protein and high molar mass AGPs and underlines their complementary role. The current study will be also helpful to optimize the formulation of a new emulsion with a different dispersed phase, taking into account that the use of limonene can be considered as a worse case.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank the Thai government and the Campus France for granting Chutima Aphibanthammakit, & ALLAND & ROBERT Company & Natural and organic gums (Port Mort, France) for their financial support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2020.100090.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.1.
Supplementary figure 1.2.

Supplementary figure 1.3.
Supplementary figure 1.4.

References
- Al-Assaf S., Phillips G.O., Williams P.A. Controlling the molecular structure of food hydrocolloids. Food Hydrocolloids. 2006;20:369–377. doi: 10.1016/j.foodhyd.2005.03.017. [DOI] [Google Scholar]
- Al-Assaf S., Phillips G.O., Aoki H., Sasaki Y. Characterization and properties of Acacia senegal (L.) Willd. var. senegal with enhanced properties (Acacia(sen) SUPER GUMTM): Part 1-Controlled maturation of Acacia senegal var. senegal to increase viscoelasticity, produce a hydrogel form and convert a poor into a good emulsifier. Food Hydrocolloids. 2007;21:319–328. doi: 10.1016/j.foodhyd.2006.04.011. [DOI] [Google Scholar]
- Alftren J., Penarietta J.M., Bergenstahl B., Nilsson L. Comparison of molecular and emulsifying properties of gum arabic and mesquite gum using asymmetrical flow field-flow fractionation. Food Hydrocolloids. 2012;26:54–62. doi: 10.1016/j.foodhyd.2011.04.008. [DOI] [Google Scholar]
- Anderson D.M.W., Weiping W. The characterization of gum arabic (Acacia senegal) samples from Uganda. Topics in Catalysis. 1991;5:297–306. doi: 10.1016/S0268-005X(09)80115-X. [DOI] [Google Scholar]
- Aoki H., Katayama T., Ogasawara T., Sasaki Y., Al-Assaf S., Phillips G.O. Characterization and properties of Acacia senegal (L.) Willd. var. senegal with enhanced properties (Acacia (sen) SUPER GUMTM): Part 5— Factors affecting the emulsification of Acacia senegal and Acacia (sen) SUPER GUMTM. Food Hydrocolloids. 2007;21:353–358. doi: 10.1016/j.foodhyd.2006.04.014. [DOI] [Google Scholar]
- Aphibanthammakit, C. (2018). Interfacial and emulsifying properties of Acacia senegal and Acacia seyal gum and their fractions. PhD Thesis. University of Montpellier.
- Apolinar-Valiente R., Williams P., Nigen M., Mejia Tamayoa V., Doco T., Sanchez C. Recovery, structure and physicochemical properties of an aggregate-rich fraction from Acacia senegal gum. Food Hydrocolloids. 2019;89:864–873. doi: 10.1016/j.foodhyd.2018.11.054. [DOI] [Google Scholar]
- Atgié M., Masbernat O., Roger K. Emulsions stabilized by gum Arabic: Composition and packing within interfacial films. Langmuir. 2019;35:962–972. doi: 10.1021/acs.langmuir.8b02715. [DOI] [PubMed] [Google Scholar]
- Bai L., Huan S., Li Z., McClements D.J. Comparison of emulsifying properties of food-grade polysaccharides in oil-in-water emulsions: Gum arabic, beet pectin, and corn fiber gum. Food Hydrocolloids. 2017;66:144–153. doi: 10.1016/j.foodhyd.2016.12.019. [DOI] [Google Scholar]
- Bai L., Huan S., Gu J., McClements D.J. Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using natural emulsifiers: Saponins, phospholipids, proteins, and polysaccharides. Food Hydrocolloids. 2016;61:703–711. doi: 10.1016/j.foodhyd.2016.06.035. [DOI] [Google Scholar]
- Castellani O., Guibert D., Al-Assaf S., Axelos M., Phillips G.O., Anton M. Hydrocolloids with emulsifying capacity. Part 1 – Emulsifying properties and interfacial characteristics of conventional (Acacia senegal (L.) Willd. var. senegal) and matured (Acacia (sen) SUPER GUM) Acacia senegal. Food Hydrocolloids. 2010;24:193–199. doi: 10.1016/j.foodhyd.2009.09.005. [DOI] [Google Scholar]
- Chanamai R., McClements D.J. Depletion flocculation of beverage emulsions by gum arabic and modified starch. Journal of Food Science. 2001;66(3):457–463. doi: 10.1111/j.1365-2621.2001.tb16129.x. [DOI] [Google Scholar]
- Dickinson E., Murray B.S., Stainsby G., Anderson D.M.W. Surface activity and emulsifying behaviour of some Acacia gums. Food Hydrocolloids. 1988;2:477–490. doi: 10.1016/S0268-005X(88)80047-X. [DOI] [Google Scholar]
- Dickinson E., Mauffret A., Rolfe S.E., Woskett C.M. Adsorption at interfaces in dairy systems. Journal of the Society of Dairy Technology. 1989;42:18–22. [Google Scholar]
- Dickinson E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocolloids. 2003;17:25–39. doi: 10.1016/S0268-005X(01)00120-5. [DOI] [Google Scholar]
- FAO (1999). Gum Arabic. Food and Nutrition Paper. In N°52, addendum 7. Rome.
- Fauconnier M.-L., Blecker C., Groyne J., Razafindralambo H., Vanzeveren E., Marlier M., Paquot M. Characterization of two Acacia gums and their fractions using a Langmuir film balance. Journal of Agricultural and Food Chemistry. 2000;48:2709–2712. doi: 10.1021/jf990749x. [DOI] [PubMed] [Google Scholar]
- Fincher G.B., Stone B.A., Clarke A.E. Arabinoglactan-proteins: Structure, biosynthesis, and function. Annual Review of Plant Physiology. 1983;34:47–70. doi: 10.1146/annurev.pp.34.060183.000403. [DOI] [Google Scholar]
- Fisher L.R., Parker N.S. Effect of surfactants on the interactions between emulsion droplets. In: Dickinson E., Stainsby G., editors. Advances in food emulsions and foams. Elsevier Appl. Sci. Publ.; London: 1988. pp. 45–90. [Google Scholar]
- Flindt C., Al-Assaf S., Phillips G.O., Williams P.A. Studies on acacia exudate gums. Part V. Structural features of Acacia seyal. Food Hydrocolloids. 2005;19:687–701. doi: 10.1016/j.foodhyd.2004.09.006. [DOI] [Google Scholar]
- Gekko K., Yamagami K. Flexibility of food proteins as revealed by compressibility. Journal of Agricultural and Food Chemistry. 1991;39:57–62. [Google Scholar]
- Gu Y.S., Decker E., McClements D.J. Influence of pH and carrageenan type on properties of b-lactoglobulin stabilized oil-in-water emulsions. Food Hydrocolloids. 2005;19:83–91. doi: 10.1016/j.foodhyd.2004.04.016. [DOI] [Google Scholar]
- Han L., Hu B., Ma R., Gao Z., Nishinari K., Phillips G.O.…Fang Y. Effect of arabinogalactan protein complex content on emulsification performance of gum arabic. Carbohydrate Polymers. 2019;224:115170–115194. doi: 10.1016/j.carbpol.2019.115170. [DOI] [PubMed] [Google Scholar]
- Huang X., Kakuda Y., Cui S.W. Hydrocolloids in emulsions: Particle size distribution and interfacial activity. Food Hydrocolloids. 2001;15:533–542. doi: 10.1016/S0268-005X(01)00091-1. [DOI] [Google Scholar]
- Islam A.M., Phillips G.O., Sljivo A., Snowden M.J., Williams P.A. A review of recent developments on the regulatory, structural and functional aspects of gum arabic. Food Hydrocolloids. 1997;11:493–505. doi: 10.1016/S0268-005X(97)80048-3. [DOI] [Google Scholar]
- Koocheki A., Taherian A.R., Razavi S.M.A., Bostan A. Response surface methodology for optimization of extraction yield, viscosity, hue and emulsion stability of mucilage extracted from Lepidium perfoliatum seeds. Food Hydrocolloids. 2009;23:2369–2379. doi: 10.1016/j.foodhyd.2009.06.014. [DOI] [Google Scholar]
- Lopez-Torrez L., Nigen M., Williams P., Doco T., Sanchez C. Acacia senegal vs. Acacia seyal gums – Part 1: Composition and structure of hyperbranched plant exudates. Food Hydrocolloids. 2015;51:41–53. doi: 10.1016/j.foodhyd.2015.04.019. [DOI] [Google Scholar]
- Mejia-Tamayo V. (2018). Propriétés volumétriques des Arabinogalactane-protéines d’exsudats de gommes d’Acacia, Ph.D., University of Montpellier, France.
- Mejia Tamayo V., Nigen M., Apolinar-Valiente R., Doco T., Williams P., Renard D., Sanchez C. Flexibility and hydration of amphiphilic hyperbranched arabinogalactan-protein from plant exudate: A volumetric perspective. Colloids and Interfaces. 2018;2:1–24. doi: 10.3390/colloids2010011. [DOI] [Google Scholar]
- Nakauma M., Funami T., Noda S., Ishihara S., Al-Assaf S., Nishinari K., Phillips G.O. Comparison of sugar beet pectin, soybean soluble polysaccharide, and gum arabic as food emulsifiers. 1. Effect of concentration, pH, and salts on the emulsifying properties. Food Hydrocolloids. 2008;22:1254–1267. doi: 10.1016/j.foodhyd.2007.09.004. [DOI] [Google Scholar]
- Osman M.E., Menzies A.R., Martin B.A., Williams P.A., Phillips G.O., Baldwin T.C. Characterization of gum Arabic fractions obtained by anion-exchange chromatography. Phytochemistry. 1995;38:409–417. doi: 10.1016/0031-9422(94)00645-A. [DOI] [Google Scholar]
- Padala S.R., Williams P.A., Phillips G.O. Adsorption of gum Arabic, egg white protein, and their mixtures at the oil-water interface in limonene oil-in-water emulsions. Journal of Agricultural and Food Chemistry. 2009;57:4964–4973. doi: 10.1021/jf803794n. [DOI] [PubMed] [Google Scholar]
- Razumovsky L., Damodaran S. Surface activity−compressibility relationship of proteins at the air−water interface. Langmuir. 1999;15:1392–1399. doi: 10.1021/la980873v. [DOI] [Google Scholar]
- Randall R.C., Phillips G.O., Williams P.A. The role of the proteinaceous component on the emulsifying properties of gum Arabic. Food Hydrocolloids. 1988;2:131–140. doi: 10.1016/S0268-005X(88)80011-0. [DOI] [Google Scholar]
- Randall R.C., Phillips G.O., Williams P.A. Fractionation and characterization of gum from Acacia senegal. Food Hydrocolloids. 1989;3:65–75. doi: 10.1016/S0268-005X(89)80034-7. [DOI] [Google Scholar]
- Ray A.K., Bird P.B., Iacobucci G.A., Clark B.C. Functionality of gum Arabic. Fractionation, characterization and evaluation of gum fractions in citrus oil emulsions and model beverages. Food Hydrocolloids. 1995;9:123–131. doi: 10.1016/S0268-005X(09)80274-9. [DOI] [Google Scholar]
- Renard D., Lavenant-Gourgeon L., Ralet M.-C., Sanchez C. Acacia senegal gum: Continuum of molecular species differing by their protein to sugar ratio, molecular weight and charges. Biomacromolecules. 2006;7:2637–2649. doi: 10.1021/bm060145j. [DOI] [PubMed] [Google Scholar]
- Sanchez C., Nigen M., Mejia Tamayo V., Doco T., Williams P., Amine C., Renard D. Acacia gum: History of the future. Food Hydrocolloids. 2018;78:140–160. doi: 10.1016/j.foodhyd.2017.04.008. [DOI] [Google Scholar]
- Trindade J.R., Freire M.G., Amaral P.F.F., Coelho M.A.Z., Coutinho J.A.P., Marrucho I.M. Aging mechanisms of oil-in-water emulsions based on a bioemulsifier produced by Yarrowia lipolytica. Colloids and Surfaces A-Physico-chemical and Engineering Aspects. 2008;32:149–154. [Google Scholar]
- Vogler E.A. Protein adsorption in three dimensions. Biomaterials. 2012;33:1201–1237. doi: 10.1016/j.biomaterials.2011.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walstra P., Oortwijn H. The membranes of recombined fat globules. 3. Mode of formation. Netherlands Milk and Dairy Journal. 1982;36:103–113. [Google Scholar]
- Xiang S., Yao X., Zhang W., Zhang K., Fang Y., Nishinari K.…Jiang F. Gum Arabic-stabilized conjugated linoleic acid emulsions: Emulsion properties in relation to interfacial adsorption behaviors. Food Hydrocolloids. 2015;48:110–116. doi: 10.1016/j.foodhyd.2015.01.033. [DOI] [Google Scholar]
- Zhang J., Peppard T.L., Reineccius G.A. Preparation and characterization of nanoemulsions stabilized by food biopolymers using microfluidization. Flavour and Fragrance Journal. 2015;30(4):288–294. doi: 10.1002/ffj.3244. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.