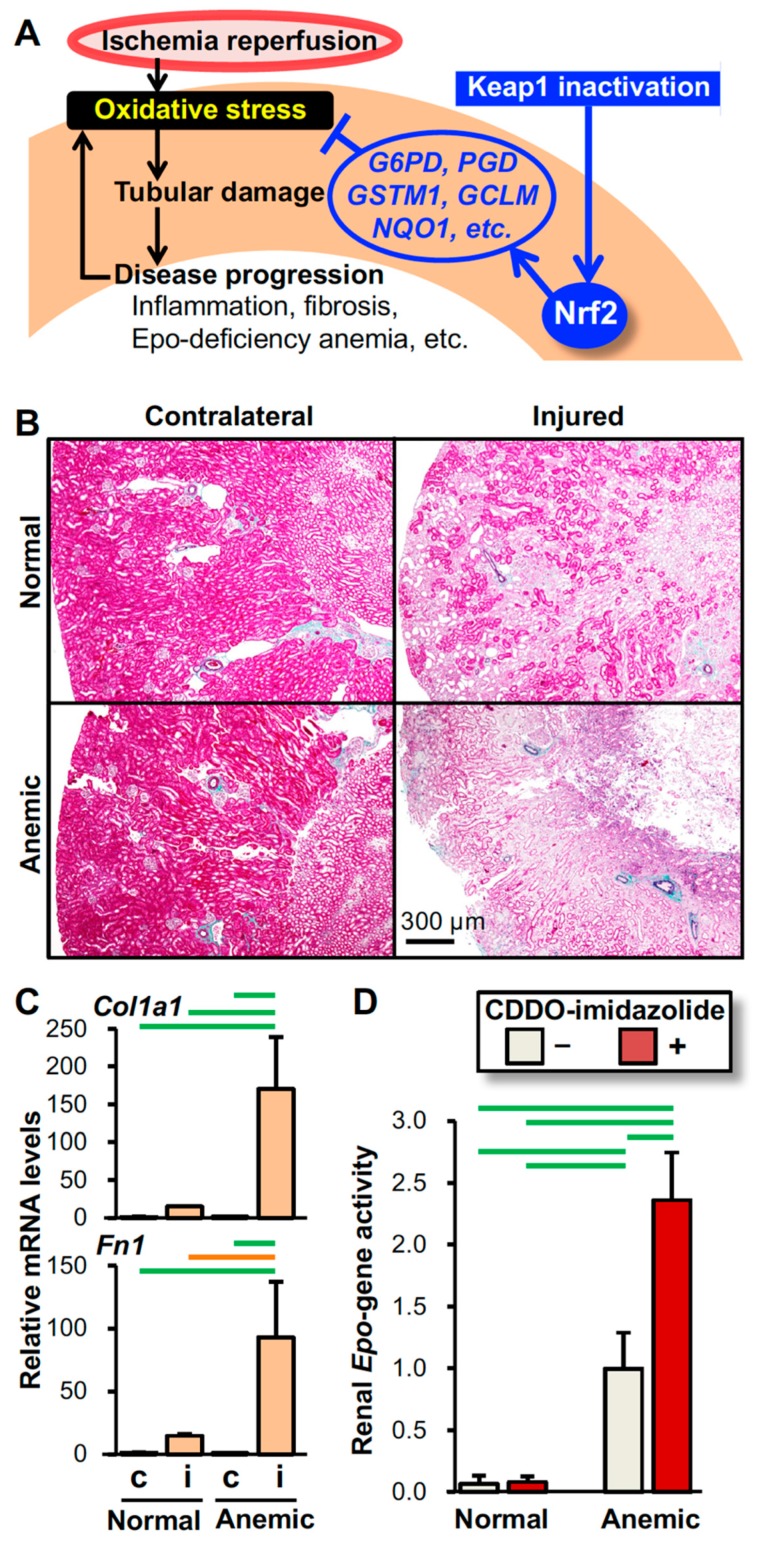
Figure 4.
Mechanism underlying the intervention by Nrf2 activation in oxidative kidney injury. (A) Schematic model showing that Nrf2 protects kidneys from IRI (see Figure 1). Pharmacological inactivation of Keap1 after IRI is effective in protecting kidneys from oxidative injury by inducing the expression of antioxidative genes, which are targets of Nrf2 (see Figure 2). (B) Epo-deficiency anemia aggravates IRI-induced damage in kidneys. Elastica-Masson staining of the injured and contralateral kidneys from the anemic and normal mice 14 days after unilateral renal IRI demonstrates that the pale pink damaged area expands in the injured kidney of the mouse model for Epo-deficiency anemia compared to that of the normal control mouse. (C) Expression of the Col1a1 and Fn1 genes (encoding type-1α1 collagen and fibronectin, respectively) in injured (i) and contralateral (c) kidneys from anemic and normal mice 14 days after unilateral renal IRI surgery demonstrates that Epo-deficiency anemia aggravates kidney fibrosis. Notably, the expression levels of these fibrosis-related genes are very low in the uninjured contralateral kidneys. (D) The Nrf2 activator CDDO-imidazolide enhances renal Epo gene activity induced by anemic conditions. CDDO-imidazolide (30 µmol/kg body weight) was orally administered to anemic and normal mice on days 1 and 3 and then, RNA samples from kidneys were prepared on day 4. Since the Epo gene was replaced with GFP (green fluorescent protein) cDNA in the anemic mice, the renal Epo gene activity was estimated with the expression levels of the GFP gene. In the control mice, the Epo gene was heterozygously replaced with GFP cDNA [111]. n = 3 (C) and n = 5 (D) per group. The data are shown as the mean with standard deviations (C and D). p < 0.01 (green line) and p < 0.05 (orange line) among the groups, determined by the Tukey-Kramer highest significance difference test (C and D).