Abstract
Residential radon exposure and cigarette smoking are the two most important risk factors for lung cancer. The combined effects thereof were evaluated in a multi-center matched case-control study in South Korea. A total of 1038 participants were included, comprising 519 non-small cell lung cancer cases and 519 age- and sex- matched community-based controls. Residential radon levels were measured for all participants. Multivariate logistic regression was used to calculate odds ratios (OR) for lung cancer according to radon exposure (high ≥ 100 Bq/m3 vs. low < 100 Bq/m3), smoking status, and combinations of the two after adjusting for age, sex, indoor hours, and other housing information. The median age of the participants was 64 years, and 51.3% were women. The adjusted ORs (95% confidence intervals [CIs]) for high radon and cigarette smoking were 1.56 (1.03–2.37) and 2.53 (1.60–3.99), respectively. When stratified according to combinations of radon exposure and smoking status, the adjusted ORs (95% CIs) for lung cancer in high-radon non-smokers, low-radon smokers, and high-radon smokers were 1.40 (0.81–2.43), 2.42 (1.49–3.92), and 4.27 (2.14–8.52), respectively, with reference to low-radon non-smokers. Both residential radon and cigarette smoking were associated with increased odds for lung cancer, and the difference in ORs according to radon exposure was much greater in smokers than in non-smokers.
Keywords: radon, cigarette smoking, lung cancer
1. Introduction
An aggressive cancer, lung cancer is the most common cause of cancer death worldwide [1]. While prognoses of lung cancer at advanced stages remain disappointing [2], survival rates for early localized disease are often promising [3], although early detection of lung cancer is uncommon. Therefore, current preventive strategies focus on controlling environmental hazards or routine radiologic screening of individuals at high risk for lung cancer [4].
The two most important environmental contributors to lung cancer development are cigarette smoking and radon exposure. Of these, exposure to radon indoors has garnered greater interest as a risk factor for lung cancer, as radon is a colorless and odorless gas that is ubiquitous in rocks and soils and, thus, can accumulate in buildings [5]. The association between radon exposure and lung cancer has been widely reported [6,7,8] and the combined effect of radon and tobacco smoke is thought to be higher than additive [9,10]. However, studies on the combined effect of radon and smoking on lung cancer have primarily focused on exposure to radon at high concentration sites, such as uranium mines [11,12,13]. Accordingly, we aimed to assess the interaction between residential radon exposure and cigarette smoking in association with lung cancer in a matched case-control study in Korea.
2. Methods
2.1. Study Design and Participants
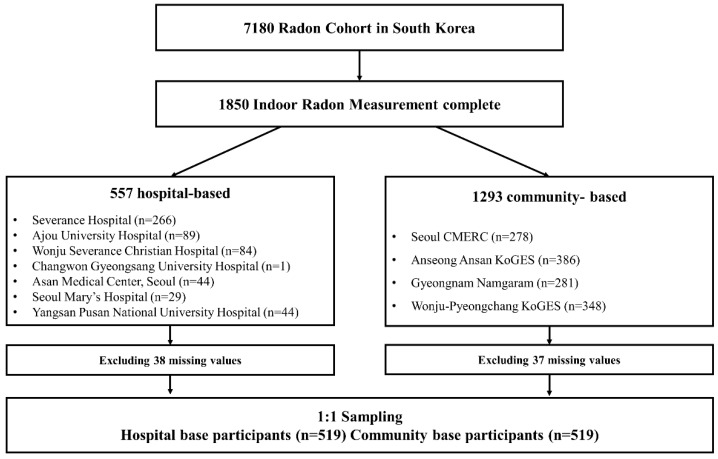
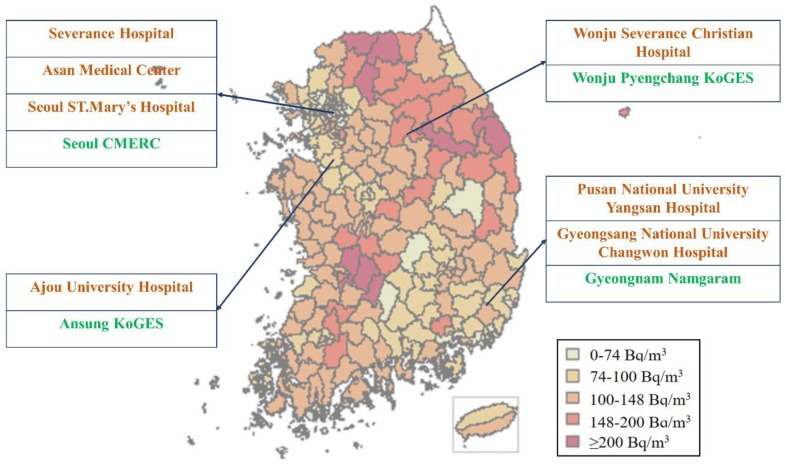
The Korea-Integrated Radon Exposure Epidemiology Statistics (K-iREES) study enrolled a total of 6582 individuals between October 2015 and March 2018 from seven tertiary hospitals and four community-based cohorts (Figure 1). The hospital-based participants were recruited from Severance Hospital, Seoul; Asan Hospital, Seoul; St. Mary’s Hospital, Seoul; Ajou University Hospital, Suwon; Wonju Severance Hospital, Wonju; Gyeongsang University Hospital, Changwon; and Pusan University Hospital, Yangsan. The community-based participants were recruited from the Cardiovascular and Metabolic Diseases Etiology Research Center (CMERC), Seoul [14]; the Ansung-Ansan Korean Genome and Epidemiology Study (KoGES) [15]; the Namgaram cohort [16], Gyeongnam; and the Wonju-Pyeongchang KoGES [15]. The study regions were selected to include various regional radon exposure levels according to data obtained from the National Institute of Environmental Research (2011–2016). The selected study regions and their corresponding exposure levels are depicted in Figure 2, with correction for seasonal variations.
Figure 1.
Flowchart of the study participants.
Figure 2.
Map of the study area and research sites. Regional indoor radon levels were obtained from the National Institute of Environmental Research (2011–2016). Fill colors correspond to radon levels in five categories. Stars designate the locations of study sites.
From the study hospitals, patients aged 19 to 80 years who had been diagnosed with non-small cell lung cancer (NSCLC) stage I to IIIa were included. The controls were selected from community-based cohort participants aged 19 to 80 years who had no known diagnosis of lung cancer. All participants had lived in their homes for 2 years or longer. A total of 1343 individuals, including 526 hospital-based and 817 community-based participants, had radon measurements taken in their homes. For each hospital-based lung cancer patient, a community-based control was matched for sex and age (<65 or ≥65 years), and 1:1 sampled using SAS proc surveyselect. Finally, 519 cases and 519 matched controls were analyzed. The study protocol was approved by the Institutional Review Board of Yonsei University College of Medicine (CR315030).
2.2. Measurement of Residential Radon Levels
Residential radon levels were measured at two locations in each study home where individuals tend to spend most of their time: the living room and the bedroom. Alpha-track detectors (Raduet Model RSV-8; Radosys Ltd., Budapest, Hungary) were used as a passive radon measuring device. The measuring devices were positioned away from household electrical appliances, windows, or sealed drawers. The measurements were made over 3 months, and the average of measurements at both locations in the house was taken. Given that indoor radon levels are highest in the winter and lowest in the summer, seasonal corrections were made with average temperature, wind speed, and other factors taken into consideration [17]. The residential radon levels were dichotomized into high (≥100 Bq/m3) or low (<100 Bq/m3) according to World Health Organization reference data [18].
2.3. Smoking History and Covariables
The K-iREES study was designed to investigate factors associated with radon exposures and related health problems. Questionnaires were used to identify demographics, health-related behaviors, such as cigarette smoking, and the characteristics of individual homes, including indoor cracks, ventilation, housing types, construction year, etc. Sleeping hours was also considered, with 70 percent of the time spent breathing through the nose during sleep or rest [19]. Cigarette smoking was defined as having smoked five or more packs in a lifetime. Second-hand smoking was defined as living together with or working in proximity to a current smoker [20,21]. Green area corresponds to forest and grassland area; agricultural space, such as rice fields, is not included in green area [22].
2.4. Statistical Analysis
Participant characteristics are reported as a mean ± standard deviation, median [interquartile range], or frequency (percent). Intergroup comparisons were conducted using t-tests for continuous variables and χ2-tests for categorical variables. We used multivariate conditional logistic regression to calculate odds ratios (OR) and 95% confidence intervals (CI) for lung cancer according to residential radon exposure (high vs. low), smoking status, and combinations of the two (low-radon dwelling non-smokers [reference], high-radon dwelling smokers, low-radon dwelling smokers, and high-radon dwelling smokers), after adjusting for second-hand smoking, sleeping hours, indoor hours, housing type, floor, presence of cracks, and green ratio [22]. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Map-visualization of radon levels was computed using R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Participant Characteristics
Descriptive statistics of the 519 hospital-based lung cancer cases and the 519 age- and sex-matched community-based controls are reported in Table 1. In both the case and control groups, the median age was 64 years, and 51.3% were women. Mean residential radon levels were 65.46 Bq/m3 and 73.75 Bq/m3 (p = 0.013) in the case and control groups, respectively. Among the cases and controls, the proportions of individuals exposed to high levels of residential radon (≥100 Bq/m3) were 13.7% and 17.7% (p = 0.007); smokers comprised 42.8% and 34.9% (p = 0.009); and second-hand smokers accounted for 46.1% and 21.2% (p < 0.001), respectively. Participants in the case group reported longer sleeping and indoor hours and were more likely to live in apartments or other multi-family houses, with a lower green ratio (all p < 0.001), although with similar building ages, than participants in the control group.
Table 1.
Characteristics of the study participants.
| Variables | Case (N = 519) | Control (N = 519) | p Value |
|---|---|---|---|
| Age, years | 64 [57–72] | 64 [59–72] | 0.116 |
| Sex, n (%) | N/A | ||
| Male | 253 (48.75) | 253 (48.75) | |
| Female | 266 (51.25) | 266 (51.25) | |
| Residential radon *, Bq/m3 | 65.46 ± 46.71 | 73.75 ± 60.21 | 0.013 |
| 48.32 [34.43–73.61] | 55.06 [37.71–82.78] | <0.001 | |
| High-radon dwelling †, n (%) | 71 (13.68) | 92 (17.73) | 0.007 |
| Cigarette smoking, n (%) | 222 (42.77) | 181 (34.87) | 0.009 |
| Tobacco consumption, n (%) | <0.001 | ||
| Never-smokers | 297 (57.23) | 338 (65.13) | |
| Light smokers (1–100 pack-years) | 9 (1.73) | 7 (1.35) | |
| Moderate smokers (100–365 pack-years) | 32 (6.17) | 34 (6.55) | |
| Heavy smokers (over 365 pack-years) | 178 (34.30) | 85 (16.38) | |
| Non-response | 3(0.58) | 55(10.60) | |
| Second-hand smoking, n (%) | 239 (46.05) | 110 (21.19) | <0.001 |
| Sleeping hours | 7.20 ± 1.83 | 6.76 ± 1.44 | <0.001 |
| Indoor hours | 15.88 ± 4.39 | 14.17 ± 3.69 | <0.001 |
| Housing type, n (%) | <0.001 | ||
| Single-family house | 178 (34.30) | 373 (71.87) | |
| Apartment | 180 (34.68) | 68 (13.10) | |
| Other multi-family dwelling | 161 (31.02) | 78 (15.03) | |
| Floor of residence | 4.76 ± 5.09 | 2.63 ± 3.69 | <0.001 |
| Presence of house crack, n (%) | 120 (23.12) | 145 (27.94) | 0.075 |
| Construction year | 1996 [1990–2003] | 1997 [1987–2005] | 0.638 |
| Green ratio | 48.09 ± 21.09 | 56.89 ± 18.54 | <0.001 |
* Corrected for seasonal variations. †Residential radon ≥ 100 Bq/m3.
3.2. Residential Radon and Cigarette Smoking on Lung Cancer
In conditional logistic regression adjusted for second-hand smoking, sleeping and indoor hours, housing type and floor, house cracks, and green ratio, the ORs (95% CIs) for high radon, cigarette smoking and heavy-smoker were 1.56 (1.03–2.37), 2.53 (1.60–3.99), 5.56(3.31–9.35) respectively (Table 2). When stratified by combinations of radon exposure and smoking status (low-radon non-smokers [reference], high-radon smokers, low-radon smokers, and high-radon smokers), the difference in ORs for lung cancer by radon exposure was much greater in smokers than in non-smokers. That is, with low-radon non-smokers as the reference group, the adjusted ORs (95% CIs) for lung cancer were 1.40 (0.81–2.43), 2.42 (1.49–3.92), and 4.27 (2.14–8.52) in high-radon non-smokers, low-radon smokers, and high-radon smokers, respectively. Similar findings were observed when we used conventional, instead of conditional, logistic regression (Table 2).
Table 2.
Associations of residential radon exposure and cigarette smoking with lung cancer.
| Variables | Case, n | Control, n | Conditional Logistic Regression | Conventional Logistic Regression | ||
|---|---|---|---|---|---|---|
| OR (95% CI) * | p-Value | OR (95% CI) † | p-Value | |||
| Residential radon | ||||||
| Low (< 100 Bq/m3) | 448 | 427 | 1.00 (reference) | 1.00 (reference) | ||
| High (≥ 100 Bq/m3) | 71 | 92 | 1.56 (1.03–2.37) | 0.037 | 1.52 (1.00–2.31) | 0.048 |
| Cigarette smoking | ||||||
| Non-smokers | 297 | 338 | 1.00 (reference) | 1.00 (reference) | ||
| Smokers | 222 | 181 | 2.53 (1.60–3.99) | <0.001 | 2.50 (1.59–3.94) | <0.001 |
| Tobacco consumption | ||||||
| Never-smoker | 297 | 338 | 1.00 (reference) | 1.00 (reference) | ||
| Light smokers | 9 | 7 | 3.05 (0.81–11.43) | 0.739 | 2.47 (0.68–8.56) | 0.797 |
| Moderate smokers | 32 | 34 | 2.65 (1.32–5.30) | 0.934 | 2.03 (1.11–3.71) | 0.847 |
| Heavy smokers | 178 | 85 | 5.56 (3.31–9.35) | <0.001 | 4.24 (2.92–6.15) | <0.001 |
| Radon and smoking | ||||||
| Low-radon non-smokers | 262 | 282 | 1.00 (reference) | 1.00 (reference) | ||
| High-radon non-smokers | 35 | 56 | 1.40 (0.81–2.43) | 0.231 | 1.40 (0.81–2.44) | 0.230 |
| Low-radon smokers | 186 | 145 | 2.42 (1.49–3.92) | <0.001 | 2.42 (1.50–3.91) | <0.001 |
| High-radon smokers | 36 | 36 | 4.27 (2.14–8.52) | <0.001 | 4.02 (2.03–7.97) | <0.001 |
* Conditional logistic regression was adjusted for second-hand smoking, sleeping hours, indoor hours, housing type, floor, presence of house cracks, and green ratio. CI, confidence interval; OR, odds ratio. † Conventional logistic regression was further adjusted for age and sex.
Furthermore, we repeated the analysis with tobacco smoke-exposure reclassified into smoke-free group (neither smoking nor being exposed to second-hand smoke) and smoke-exposed group (active smoking and/or being exposed to second-hand smoke). Compared with the low-radon smoke-free group, the adjusted ORs for lung cancer in high-radon smoke-free, low-radon smoke-exposed, high-radon smoke-exposed groups were 1.01 (0.49–2.07), 2.39 (1.48–3.87), and 4.93 (2.57–9.45) from a conditional logistic model and 1.04 (0.51–2.13), 2.41 (1.49–3.89), and 4.65 (2.44–8.88) from a conventional logistic model, respectively (Table 3).
Table 3.
Associations of residential radon and tobacco smoke exposure with lung cancer.
| Variables | Case, n | Control, n | Conditional Logistic Regression | Conventional Logistic Regression | ||
|---|---|---|---|---|---|---|
| OR (95% CI) * | p-Value | OR (95% CI) † | p-Value | |||
| Residential radon | ||||||
| Low (<100 Bq/m3) | 448 | 427 | 1.00 (reference) | 1.00 (reference) | ||
| High (≥100 Bq/m3) | 71 | 92 | 1.56 (1.03–2.37) | 0.037 | 1.52 (1.00–2.31) | 0.048 |
| Smoke exposure | ||||||
| Smoke-free | 122 | 254 | 1.00 (reference) | 1.00 (reference) | ||
| Smoke-exposed | 397 | 265 | 2.67 (1.69–4.21) | <0.001 | 2.64 (1.68–4.17) | <0.001 |
| Radon and smoke exposure | ||||||
| Low-radon smoke-free | 109 | 204 | 1.00 (reference) | 1.00 (reference) | ||
| High-radon smoke-free | 13 | 50 | 1.01 (0.49–2.07) | 0.956 | 1.04 (0.51–2.13) | 0.919 |
| Low-radon smoke-exposed | 339 | 223 | 2.39 (1.48–3.87) | <0.001 | 2.41 (1.49–3.89) | <0.001 |
| High-radon smoke-exposed | 58 | 42 | 4.93 (2.57–9.45) | <0.001 | 4.65 (2.44–8.88) | <0.001 |
* Adjusted for second-hand smoking, sleeping hours, indoor hours, housing type, floor, presence of house cracks, and green ratio. CI, confidence interval; OR, odds ratio. † Conventional logistic regression was further adjusted for age and sex.
Finally, we checked the robustness of our data using a lower radon cut-off value of 74 Bq/m3 [23]. The adjusted ORs were 1.55 (1.02–2.34), 2.39 (1.45–3.95), and 4.16 (2.29–7.57) in high-radon non-smokers, low-radon smokers, and high-radon smokers, respectively (Table S1), and were comparable with ORs from the main analyses.
4. Discussion
In this matched case-control study, we discovered significant associations for lung cancer with residential radon exposure, with cigarette smoking, and with combinations of the two. Residential radon exposure and cigarette smoking were synergistically associated with a greater odds for lung cancer. Although addictive interaction (p = 0.344) and multiplicative interaction (p = 0.367) did not reach statistical significance, the difference in ORs for lung cancer according to radon exposure was much greater in current smokers than in non-smokers. Such trend was more pronounced when environmental smoking was taken into account. In this regard, for both smoking- and radon-related lung cancer risk, the most important risk reduction strategy would be smoking cessation and avoidance of environmental tobacco smoke. Conversely, among active or secondhand smokers, residential radon assessment and control should constitute a significant portion of lung cancer preventive measures, in addition to efforts supporting cessation and avoidance.
Even at concentrations far below the official guidance level, radon can lead to a 2.5 times increase in lung cancer risk. Furthermore, synergies found between smoking and radon can be useful in writing public health recommendations [24]. The analysis was conducted based on 1pCi/litter. We conducted the analysis based on WHO recommendation standard of 100Bq/m3 and 74Bq/m3.
Compared to the subjects studied in Spain, there are more packs of cigarettes consumed per year in Korea. Most of the subjects smoked a pack of cigarettes a day. The survey did not reveal the exact amount of cigarette consumption [25]. In the case of Heavy Smoker, the OR value was 3.38 (1.35–8.47) (p = 0.009) based on Radon 100Bq/m3. In the case of smoking, additional research is need on Never Smoker because it affects lung cancer more than Radon. Odds ratio were lower due to the higher proportion of non-smoking subjects and women than previous studies.
The carcinogenicity of radon and cigarette smoke may involve various mechanisms, including generation of DNA-reactive products, chromosomal instability and aberrations, and mutations of tumor-suppressor genes [26]. However, the current literature is, as of yet, inconsistent on mutation “hotspots” or unique cytogenetic markers associated with radon-related carcinogenicity or its interactions with tobacco smoke [27]. Some in vitro studies have suggested a synergistic increase of chromosomal aberrations and possibly a higher susceptibility to radon exposure in lymphocytes of smokers [28,29]. It has also been proposed that radon progeny may attach to tobacco smoke aerosols and increase potential doses to target organs [30,31]. Further molecular and cytogenetic studies are needed to elucidate the mechanism underlying the observed synergism between low-dose radon and smoking in association with lung cancer.
Epidemiologic evidence of interactions between radon exposure and cigarette smoking and their effects on lung cancer has been described in a number of studies [10,32,33]. However, many of these studies included persons exposed to a high doses of radon, such as those face by uranium miners [34,35,36] Considering the non-linear dose-response relationship between radon and lung cancer, the modifying effect of low-dose radon on the smoking-lung cancer relationship may not be extrapolated from uranium miner results. In this study, we evaluated the interaction between residential radon and cigarette smoking, and our findings hold notable implications in lung cancer risk assessment and preventive measures. Furthermore, this is the first study in Korea to describe interactions between residential radon and cigarette smoking in association with lung cancer.
Our study has several limitations. First, the case-control design precludes causal inference between the exposure variables and lung cancer. Second, although we incorporated a matched case-control design and further adjustments for other imbalances, residual and unmeasured confounding may exist. Third, the number of female smokers in our study was too small for sex-specific analyses to be possible. Fourth, recall bias in smoking history is also possible. Finally, histopathologic subtypes of NSCLC were not differentiated in our study. Notwithstanding, this study also has some notable strengths. Foremost, we used individual-level residential radon measurements rather than ecologic data. Moreover, the cases and controls were gathered from multiple centers and cohorts of different geographic locations with varying regional radon levels. Therefore, our findings may provide some generalizability on radon exposure patterns and their associations with lung cancer in Korea.
In conclusion, we found both residential radon and cigarette smoking to be associated with increased odds for lung cancer, and the difference in ORs according to radon exposure was much greater in smokers than in non-smokers. Therefore, preventive strategies targeting radon-related lung cancer should emphasize, in addition to radon-reducing repairs and ventilation, both smoking cessation and withdrawing from second-hand smoking.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/17/8/2946/s1, Table S1. Associations of residential radon exposure (≥74 Bq/m3) and cigarette smoking with lung cancer.
Author Contributions
Conceptualization, C.-M.L. and D.R.K.; Funding acquisition, D.R.K.; Formal analysis, E.J.P. and H.L.; Methodology, E.J.P. and D.R.K.; Resources, C.-M.L.; Data curation, H.C.K., S.S.S., S.B.K., K.S.P. and N.H.C.; Writing–original draft, H.L. and E.J.P.; Writing–review &editing, H.C.K. and D.R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Korean Ministry of Environment as part of the “Environmental Health Action Program” (grant number 2015001350002).
Conflicts of Interest
The authors declare no conflict of interest
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P., Chansky K., Crowley J., Rami-Porta R., Asamura H., Eberhardt W.E., Nicholson A.G., Groome P., Mitchell A., Bolejack V. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 3.International Early Lung Cancer Action Program Investigators. Henschke C.I., Yankelevitz D.F., Libby D.M., Pasmantier M.W., Smith J.P., Miettinen O.S. Survival of patients with stage I lung cancer detected on CT screening. N. Engl. J. Med. 2006;355:1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 4.McKee B.J., Hashim J.A., French R.J., McKee A.B., Hesketh P.J., Lamb C.R., Williamson C., Flacke S., Wald C. Experience with a CT screening program for individuals at high risk for developing lung cancer. J. Am. Coll. Radiol. 2015;12:192–197. doi: 10.1016/j.jacr.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer . Monographs on the Evaluation of Carcinogenic Risk to Humans: Man-Made Mineral Fibres and Radon. International Agency for Research on Cancer; Lyon, France: 1988. [Google Scholar]
- 6.Alavanja M.C., Brownson R.C., Lubin J.H., Berger E., Chang J., Boice J.D., Jr. Residential radon exposure and lung cancer among nonsmoking women. J. Natl. Cancer Inst. 1994;86:1829–1837. doi: 10.1093/jnci/86.24.1829. [DOI] [PubMed] [Google Scholar]
- 7.Auvinen A., Makelainen I., Hakama M., Castren O., Pukkala E., Reisbacka H., Rytomaa T. Indoor radon exposure and risk of lung cancer: A nested case-control study in Finland. J. Natl. Cancer Inst. 1996;88:966–972. doi: 10.1093/jnci/88.14.966. [DOI] [PubMed] [Google Scholar]
- 8.National Research Council . Health Effects of Exposure to Radon: BEIR VI. Volume 6. National Academies Press; Washington, DC, USA: 1999. [PubMed] [Google Scholar]
- 9.Tomasek L. Lung cancer risk from occupational and environmental radon and role of smoking in two Czech nested case-control studies. Int. J. Environ. Res. Public Health. 2013;10:963–979. doi: 10.3390/ijerph10030963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohm R., Sedlak A., Bulko M., Holy K. Use of threshold-specific energy model for the prediction of effects of smoking and radon exposure on the risk of lung cancer. Radiat. Prot. Dosim. 2014;160:100–103. doi: 10.1093/rpd/ncu059. [DOI] [PubMed] [Google Scholar]
- 11.Saccomanno G., Huth G.C., Auerbach O., Kuschner M. Relationship of radioactive radon daughters and cigarette smoking in the genesis of lung cancer in uranium miners. Cancer. 1988;62:1402–1408. doi: 10.1002/1097-0142(19881001)62:7<1402::AID-CNCR2820620727>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 12.Leuraud K., Billon S., Bergot D., Tirmarche M., Caer S., Quesne B., Laurier D. Lung cancer risk associated to exposure to radon and smoking in a case-control study of French uranium miners. Health Phys. 2007;92:371–378. doi: 10.1097/01.HP.0000252259.72683.2a. [DOI] [PubMed] [Google Scholar]
- 13.Amabile J.C., Leuraud K., Vacquier B., Caer-Lorho S., Acker A., Laurier D. Multifactorial study of the risk of lung cancer among French uranium miners: Radon, smoking and silicosis. Health Phys. 2009;97:613–621. doi: 10.1097/01.HP.0000363842.62922.58. [DOI] [PubMed] [Google Scholar]
- 14.Shim J.S., Song B.M., Lee J.H., Lee S.W., Park J.H., Choi D.P., Lee M.H., Ha K.H., Kim D.J., Park S., et al. Cardiovascular and Metabolic Diseases Etiology Research Center (CMERC) cohort: Study protocol and results of the first 3 years of enrollment. Epidemiol. Health. 2017;39:e2017016. doi: 10.4178/epih.e2017016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y., Han B.G., KoGES Group Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017;46:e20. doi: 10.1093/ije/dyv316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo J.I., Park J.S., Kim R.B., Seo A.R., Park Y.J., Kim M.J., Park K.S. WHO disability assessment schedule 2.0 is related to upper and lower extremity disease-specific quality of life. Qual. Life Res. 2018;27:2243–2250. doi: 10.1007/s11136-018-1869-5. [DOI] [PubMed] [Google Scholar]
- 17.Park J.H., Lee C.M., Lee H.Y., Kang D.R. Estimation of Seasonal Correction Factors for Indoor Radon Concentrations in Korea. Int. J. Environ. Res. Public Health. 2018;15:2251. doi: 10.3390/ijerph15102251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angell W.J., Zeeb H., Shannon F. WHO Handbook on Indoor Radon: A Public Health Perspective. WHO; Geneva, Switzerland: 2009. [PubMed] [Google Scholar]
- 19.Marsh J., Birchall A. Sensitivity analysis of the weighted equivalent lung dose per unit exposure from radon progeny. Radiat. Prot. Dosim. 2000;87:167–178. doi: 10.1093/oxfordjournals.rpd.a032993. [DOI] [PubMed] [Google Scholar]
- 20.Gundersen D.A., Delnevo C.D., Wackowski O. Exploring the relationship between race/ethnicity, menthol smoking, and cessation, in a nationally representative sample of adults. Prev. Med. 2009;49:553–557. doi: 10.1016/j.ypmed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Sulsky S.I., Fuller W.G., Van Landingham C., Ogden M.W., Swauger J.E., Curtin G.M. Evaluating the association between menthol cigarette use and the likelihood of being a former versus current smoker. Regul. Toxicol. Pharmacol. 2014;70:231–241. doi: 10.1016/j.yrtph.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Lee H.Y., Park J.H., Lee C.-M., Kang D.R. Affected model of indoor radon concentrations based on lifestyle, greenery ratio, and radon levels in groundwater. J. Health Inform. Stat. 2017;42:309–316. doi: 10.21032/jhis.2017.42.4.309. [DOI] [Google Scholar]
- 23.Kim S.H., Koh S.B., Lee C.M., Kim C., Kang D.R. Indoor Radon and Lung Cancer: Estimation of Attributable Risk, Disease Burden, and Effects of Mitigation. Yonsei Med. J. 2018;59:1123–1130. doi: 10.3349/ymj.2018.59.9.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barros-Dios J.M., Barreiro M.A., Ruano-Ravina A., Figueiras A. Exposure to residential radon and lung cancer in Spain: A population-based case-control study. Am. J. Epidemiol. 2002;156:548–555. doi: 10.1093/aje/kwf070. [DOI] [PubMed] [Google Scholar]
- 25.Barros-Dios J.M., Ruano-Ravina A., Perez-Rios M., Castro-Bernardez M., Abal-Arca J., Tojo-Castro M. Residential radon exposure, histologic types, and lung cancer risk. A case-control study in Galicia, Spain. Cancer Epidemiol. Biomark. Prev. 2012;21:951–958. doi: 10.1158/1055-9965.EPI-12-0146-T. [DOI] [PubMed] [Google Scholar]
- 26.Alavanja M.C. Biologic damage resulting from exposure to tobacco smoke and from radon: Implication for preventive interventions. Oncogene. 2002;21:7365–7375. doi: 10.1038/sj.onc.1205798. [DOI] [PubMed] [Google Scholar]
- 27.Robertson A., Allen J., Laney R., Curnow A. The cellular and molecular carcinogenic effects of radon exposure: A review. Int. J. Mol. Sci. 2013;14:14024–14063. doi: 10.3390/ijms140714024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohanku M.N., Meenakshi C. Radon-induced Chromosome Damage in Blood Lymphocytes of Smokers. Res. J. Environ. Toxicol. 2012;6:51–58. doi: 10.3923/rjet.2012.51.58. [DOI] [Google Scholar]
- 29.Meenakshi C., Mohankumar M.N. Synergistic effect of radon in blood cells of smokers—An in vitro study. Mutat. Res. 2013;757:79–82. doi: 10.1016/j.mrgentox.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Morawska L., Phillips C.R. Attachment of Radon Progeny to Cigarette-Smoke Aerosol. Aerosol Sci. Technol. 1992;17:149–158. doi: 10.1080/02786829208959567. [DOI] [Google Scholar]
- 31.Biermann A.H., Sawyer S.R. Attachment of Radon Progeny to Cigarette-Smoke Aerosol (No. UCRL-CR--120647.)s. Lawrence Livermore National Laboratory; Livermore, CA, USA: 1995. [Google Scholar]
- 32.Kreuzer M., Walsh L., Schnelzer M., Tschense A., Grosche B. Radon and risk of extrapulmonary cancers: Results of the German uranium miners′ cohort study, 1960–2003. Br. J. Cancer. 2008;99:1946–1953. doi: 10.1038/sj.bjc.6604776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denman A.R., Rogers S., Ali A., Sinclair J., Phillips P.S., Crockett R.G., Groves-Kirkby C.J. Small area mapping of domestic radon, smoking prevalence and lung cancer incidence—A case study in Northamptonshire, UK. J. Environ. Radioact. 2015;150:159–169. doi: 10.1016/j.jenvrad.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Hornung R.W., Meinhardt T.J. Quantitative risk assessment of lung cancer in U.S. uranium miners. Health Phys. 1987;52:417–430. doi: 10.1097/00004032-198704000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Tomasek L., Darby S.C., Swerdlow A.J., Placek V., Kunz E. Radon exposure and cancers other than lung cancer among uranium miners in West Bohemia. Lancet. 1993;341:919–923. doi: 10.1016/0140-6736(93)91212-5. [DOI] [PubMed] [Google Scholar]
- 36.Heidenreich W.F., Tomasek L., Rogel A., Laurier D., Tirmarche M. Studies of radon-exposed miner cohorts using a biologically based model: Comparison of current Czech and French data with historic data from China and Colorado. Radiat. Environ. Biophys. 2004;43:247–256. doi: 10.1007/s00411-004-0266-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.