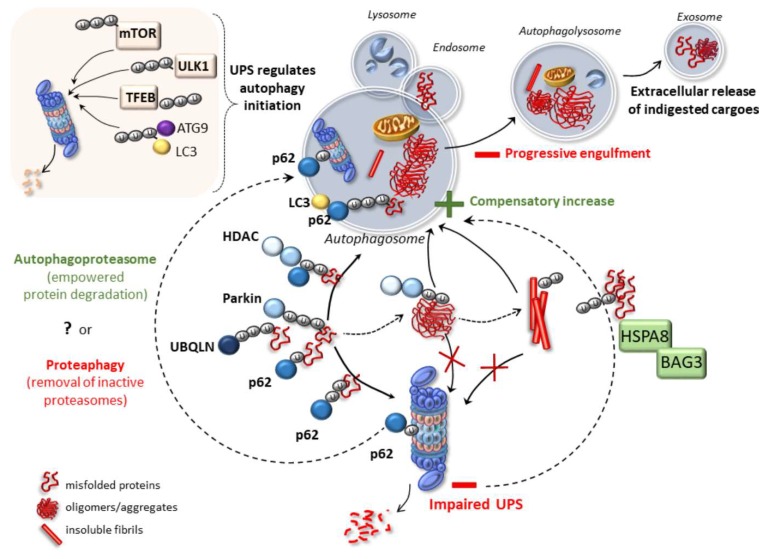
Figure 1.
Crosstalk mechanisms between ubiquitin proteasome system (UPS) and autophagy. Ubiquitin tagging is key in sorting misfolded proteins for either UPS- or autophagy-dependent degradation. Several proteins, including UBQLNs, Parkin and SQSTM1/p62, link ubiquitinated misfolded/aggreagted proteins to either UPS or autophagy. Besides misfolded substrates, p62 is key in shuttling the same proteasome to autophagy vacuoles. Functionally, the merging of UPS with autophagy vacuoles may underlie either the formation of a cell-clearing organelle endowed with empowered clearing capacity (the autophagoproteasome), or to the degradation of inactive UPS subunits (proteaphagy). Complementarily, the UPS may control autophagy dynamics by orchestrating the turnover of autophagy-related proteins (LC3 and ATG9) and protein kinases which are implicated in autophagy initiation (mTOR, ULK1 and TFEB). Parkin couples target proteins and mitochondria with dynein motor complexes via the HDAC6 to facilitate their transport towards autophagy compartments. HDAC6 activity is essential for autophagy to compensate for protein degradation when UPS is impaired. This is key when dealing with large oligomers or insoluble fibrils which may occlude the UPS. A UPS impairment may trigger a compensatory increase in autophagy activity. This occurs following the recruitment of HSPA8-BAG3 proteins which reroute protein substrates towards the autophagy pathway. Nonetheless, the accumulation of large, insoluble protein aggregates including oligomers and fibrils, may eventually engulf the autophagy pathway, leading to a prion-like, cell-to-cell propagation of indigested proteins through exosomes release.