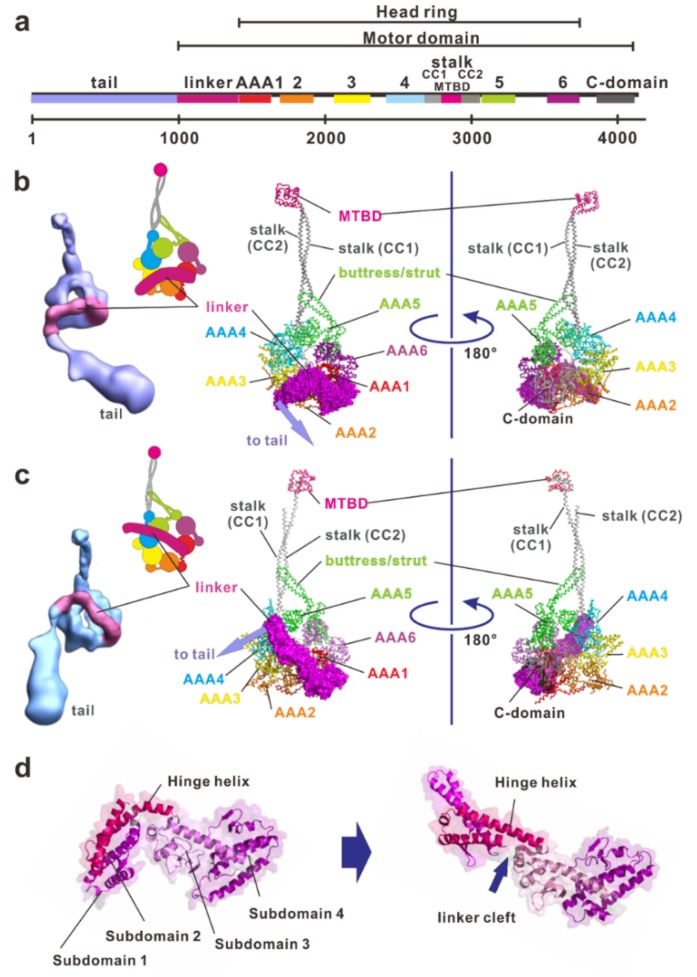
Figure 2.
The structure and molecular organization of a dynein. (a) Linear map of the heavy chain of Chlamydomonas inner arm dynein c (BAE19786). (b) The electron density 3D map of the native axonemal dynein c in ADP-vanadate (Vi) state [54] and atomic structure of the dynein-2 motor domain in ADP-Vi state (PDB ID: 4RH7). Concatenated AAA+ modules form a ring, which are indicated in red, orange, yellow, cyan, green, and purple for AAA1 through AAA6, respectively. Inset, a schematic drawing of the dynein heavy chain in the ADP-Vi state. The large and small domains of each AAA+ module are depicted using large and small circles, respectively. (c) Electron density 3D map of the whole molecule of the native axonemal dynein c in the apo state (no-nucleotide state) [54]. Atomic structure of the dynein motor domain in the ADP state (PDB ID: 3VKH). A part of the stalk is missing in this structure because of the low electron densities. Inset, a schematic drawing of the dynein heavy chain in the apo state. (d) Structural changes of the linker. From N-terminus, subdomain 1, 2, 3, and 4 can be identified. Subdomain 4 is connected into AAA1. In the apo state, the subdomain 1 connects with AAA5, but in the ADP state it connects with with AAA4. The subdomains 1 and 2 are mobile and undergo a rigid-body rotation relative to the static subdomains 3 and 4. These figures were prepared using PyMOL provided by DeLano Scientific LLC (http://www.pymol.org).