Abstract
Hormones and their receptors play an important role in the development and progression of breast cancer. Hormones regulate the proliferation of breast cancer cells through binding between estrogen or progestins and steroid receptors that may reside in the cytoplasm or be transcriptionally activated as steroid–protein nuclear receptor complexes. However, receptors for nonpeptide hormones also exist in the plasma membrane. Via those receptors, hormones are able to stimulate breast cancer cell proliferation when activated. Integrins are heterodimeric structural proteins of the plasma membrane. Their primary functions are to interact with extracellular matrix proteins and growth factors. Recently, integrin αvβ3 has been identified as a receptor for nonpeptide hormones, such as thyroid hormone and dihydrotestosterone (DHT). DHT promotes the proliferation of human breast cancer cells through binding to integrin αvβ3. A receptor for resveratrol, a polyphenol stilbene, also exists on this integrin in breast cancer cells, mediating the anti-proliferative, pro-apoptotic action of the compound in these cells. Unrelated activities of DHT and resveratrol that originate at integrin depend upon downstream stimulation of mitogen-activated protein kinase (MAPK, ERK1/2) activity, suggesting the existence of distinct, function-specific pools of ERK1/2 within the cell. This review will discuss the features of these receptors in breast cancer cells, in turn suggesting clinical applications that are based on the interactions of resveratrol/DHT with integrin αvβ3 and other androgen receptors.
Keywords: integrin αvβ3, dihydrotestosterone, resveratrol, breast cancer
1. Introduction
The theory that hormone binding to the cell surface can contribute to breast carcinogenesis was developed in large part from the discovery of plasma membrane estrogen receptors [1]. Integrins are heterodimeric structural proteins of the plasma membrane. Their primary roles are to promote cell–cell adhesion and the interactions of cells with extracellular matrix proteins. Integrin αvβ3 is expressed by cancer cells, as well as rapidly dividing endothelial and vascular smooth muscle cells. Recently, distinct receptors on the plasma membrane integrin αvβ3 of breast cancer (BC) cells have been described for thyroid hormone, resveratrol, and dihydrotestosterone (DHT). Integrins have been generally seen to bear receptors or binding sites only for relatively large molecules—extracellular matrix proteins and growth factors [2,3,4]—and thus it was surprising to find apparently biologically relevant binding sites for small molecules on integrins. Functions of these membrane receptors include modulating cancer cell proliferation and, in the case of the thyroid hormone, tumor-relevant angiogenesis.
Estrogen receptor (ER)–progesterone receptor (PR) status in breast carcinomas has three molecular subtypes: human epidermal growth factor receptor 2 (HER2) over-expression, ER and PR expression and absence of ER, and PR and HER2 expression (triple negative). These receptors may be considered targets for management of breast cancer proliferation. Recently, Giovannelli et al. also reported the role of sex steroid receptors (SSRs) in BC stem cells (BCSCs); although the growth and metastatic regulation of BCSCs is still unclear, SSRs expressed in BCSCs are considered as a marker of stemness and are linked to BC proliferation, as well as metastatic and malignant properties [5]. Overexpression of SSRs in BC usually suggests that their role is in the tumor microenvironment and the levels of circulating sex hormones; however, the SSRs’ function in BC is still conflicting [6]. In this review, we will describe the features of these receptors in breast cancers and propose clinical applications based on the interactions of resveratrol/DHT and integrin αvβ3, as well as other androgen receptors. The effects of steroid hormones and thyroid hormone and their receptors on cancer growth are summarized in Table 1.
Table 1.
| Hormone | Receptor | Functions | References |
|---|---|---|---|
| Estrogen | Estrogen Receptor-α (ER-α) | To form ligand-ER complex and controlling gene expression. To stimulate proliferation of breast cancer cells |
[7] |
| Integrin αvβ3 | NA | [8] | |
| DHT | Androgen Receptor (AR) | To form ligand-AR complex and controlling gene expression To stimulate proliferation of prostate cancer cells. |
[9] |
| Estrogen Receptor-α (ER-α) | To stimulate proliferation of ER-positive breast cancer cells | [7] | |
| Integrin αvβ3 | To stimulate proliferation of ER-negative breast cancer cells | [10] | |
| Thyroid hormone | Thyroid hormone Receptor-α (TR-α) | To stimulate cancer cell growth | [11] |
| Thyroid hormone Receptor-β (TR-β) | To inhibit cancer cell growth, however, mutant TR-β may activate cancer cell growth | [12] | |
| Integrin αvβ3 | To stimulate cancer cell growth | [13] |
2. Androgen and Other Non-Peptide Hormones Act via Different Receptors to Induce Proliferation of Human Breast Cancer Cells
In the traditional concept of steroid action, ERs and androgen receptors (ARs) exist in the cytosol and translocate into the nucleus after complexing with their respective ligands. The androgen–AR complex binds to the promoter of androgen-responsive genes. However, there is evidence for the existence of more than one cellular androgen binding site. The relative roles of ER and AR in breast cancer proliferation are controversial. For example, dehydroepiandrosterone sulfate (DHEAS) causes breast cancer cell proliferation via ERs, but can also inhibit proliferation through ARs [14,15].
The AR can be an important contributor to breast cancer cell proliferation, as Yeh et al. have shown [16]. Up to 85% of breast cancers express nuclear ARs [16]. In addition, 25%–82% of metastatic breast tumors that are ER- and PR-negative still express a significant number of ARs [16]. Most ER-positive breast tumors express ARs [15,17,18], and AR expression in ER-positive cases is associated with smaller tumor size, lower Nottingham grade, and less frequent tumor cell necrosis [19]. AR is also expressed in ER-negative/progesterone receptor (PR)-negative/HER2+ tumors, and in a subset of triple-negative apocrine tumors [19]. Approximately one in five breast cancer 1 (BRCA1) gene-expressing cancers also express Ars, but are negative for ERs and PRs [20]. However, our studies indicate that the detectable ARs in ER-negative MDA-MB-231 cells may not be functional for DHT, since there is no proliferation by DHT in the presence or absence of AR siRNA [7].
Steroid hormones and their receptors are implicated in the pathogenesis of breast cancers [21]. Studies further indicate that the interaction of the AR–ligand and co-activator plays an important role in gene expression. The AR co-activator, p44/Mep50, a subunit of the methylosome complex, enhances AR-mediated transcription activity in a ligand-dependent manner [22,23]. While it may act as a nuclear co-activator in breast cancer cells, p44 is also present in substantial quantities in the cytoplasm of terminal ductal lobular units [22,23]. When overexpressed by MCF7 breast cancer cells, p44 has been shown to enhance proliferation and invasiveness [22].
The other nuclear receptor co-activator related to invasion is actin-binding protein, actinin α 4 (ACTN4), which has been shown to promote the proliferation of MCF-7 breast cancer cells [24]. Knockdown of ACTN4 reduces transcription of ERα target genes and modulates MCF-7 cell proliferation in the absence of estrogen [24]. In late-stage metastatic breast cancers, the ACTN4 levels decrease in the nucleus, as is observed in high-grade cancerous prostate samples, suggesting that ACTN4 is possibly deregulated in advanced stage cancers [25]. ACTN4 and protein kinase C δ (PKC δ) display both co-activator and co-repressor activity in the process of AR-mediated transcription, whereas clathrin heavy chains exhibit co-activator activity during AR-mediated transcription [25].
3. Membrane Androgen Receptors
Breast and prostate cancer cells express membrane androgen receptors (mARs) subject to control by specific ligands. A panel of essential functions of these cells—proliferation, cell motility, and susceptibility to apoptosis—is regulated by such ligands [26,27] mAR-linked actions can readily be distinguished from those initiated or mediated by classical intracellular androgen receptors (iARs) by certain anti-androgens [27]. Testosterone analogues that are excluded from the cell interior, e.g., testosterone–bovine serum albumin (BSA), may express significant androgen-related biologic actions in breast cancer cells that contain iARs [28,29]. Stimulation of colon mAR by the testosterone–BSA conjugate induces rapid cytoskeleton reorganization and apoptotic responses, even in the presence of anti-androgens [26]. Apoptosis that is testosterone-induced is related to p38 MAPK and phosphatidylinositol-3-kinase (PI-3K)/Akt/NF-κB or Rho/actin pathways. The JNK/c-JUN signaling pathway appears to mediate certain iAR-initiated events [29]. The non-permeable testosterone–BSA conjugate binds mARs to stimulate early actin reorganization, which is regulated by early phosphorylation of focal adhesion kinase (FAK) and subsequent PI-3K and Rac1 activation.
Acting on breast fibroblasts in vitro, testosterone has been shown by Quinn and co-workers to enhance estrogen-responsive pS2 gene transcription and the generation of estradiol via aromatase activity in the medium [30]. Addition of an aromatase inhibitor blocked production of fibroblast-source estrogen and modestly increased cell pS2 transcription [30]. It is not yet clear what the clinical significance may be of the AR on the cell surface of breast cancer cells. However, when the action of DHT on ER-α-positive breast cancer MCF-7 cells is examined, the androgen stimulates cell proliferation. Treatment with an ERα antagonist, ICI 182,780, and siRNA knockdown of ER blocked the proliferative effect of DHT on MCF-7 cells [7]. These results suggest that DHT stimulates MCF-7 cell proliferation via ERα rather than via an AR.
4. Integrin αvβ3 as a Receptor for DHT
Although androgen may inhibit the proliferation of breast cancer cells [31,32,33], a stimulatory effect of DHT on the proliferation of triple-negative human breast cancer MDA-MB-231 cells has been observed [7]. Integrin monomer αv antibodies and Arg-Gly-Asp (RGD) peptides inhibit the action of DHT in MDA-MB-231 cells, but are ineffective in MCF-7 cells [7]. Thus, the mechanisms of DHT action differ in ER-positive and -negative breast cancer cell lines, and only in the ER-negative cell lines is there evidence for the existence of a DHT receptor on integrin αvβ3. Studied in prostate cancer and breast cancer cells, ligand-binding to integrin αvβ3 activates FAK, and consequently, FAK, PI-3K, and the Rac1 pathway, leading to the reorganization of actin [34].
Increased FAK activity in tumors has been shown to contribute to phosphorylation of Shc and likely to the promotion of Ras activity, extracellular signal-regulated kinase 2 (ERK2) activation, and cell proliferation in vitro and in vivo [35]. Evidence also indicates that recruitment of an isoform of Shc adaptor proteins, p66Shc, is linked to integrin αvβ3 clustering [35,36,37]. The levels of p66Shc are higher in cancer cells than that in the adjacent non-malignant cells in breast, prostate, ovarian, thyroid, and colon carcinoma tissues [38]. Prostate and ovarian cancer cell proliferation appear to require functional steroid receptors and the elevation of p66Shc protein levels [39].
On the other hand, DHT binds to integrin αvβ3 and stimulates ERα-negative breast cancer proliferation, in which phosphorylation of integrin αvβ3-associated p66Shc is either stimulated by DHT directly or indirectly via the vascular endothelial growth factor (VEGF) signal pathway. In these steroid-treated cells, the level of p66Shc protein is elevated, at least in part due to the inhibition of its ubiquitination [39]. This suggests the existence of a possible therapeutic pathway via the upregulation of ubiquitination of p66Shc protein in advanced cancers.
5. Androgens and Breast Cancer Cell Proliferation
Whether androgens are able to induce breast cancer cell proliferation has been a matter of debate. The aromatase activity of breast cancer cells may be sufficient to convert androgen to estrogen and generate local estrogen responses [40]. This process may require the complexation of aromatase and cytochrome P450. This testosterone-induced response of the expression of estrogen-responsive gene pS2 is inhibited by the aromatase inhibitor 7α (4′-amino) phenylthio-1,4-androstadiene-3,17-dione (7α-APTADD) and by 10 µM tamoxifen in breast cancer MCF-7 cells [41]. In the patient on tamoxifen or an aromatase inhibitor who has a recurrent ER-α-positive tumor, it is possible that residual circulating androgen is contributing to breast cancer cell proliferation [42]. To address this issue, the androgen analog specificity of the DHT receptor needs to be determined.
In addition to aromatase pathway, the sulfatase pathway converts estrone sulfate (E1S) into estrone (E1) and into final product E2, synthesized by the 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1). The molecular mechanisms of 17β-HSD1-induced breast cancer growth include estradiol synthesis and DHT inactivation. In addition, 17β-HSD1 can enhance the E2-induced expression of endogenous pS2; this suggests involvement of 17β-HSD1 in estrogen responsiveness and breast cancer growth [43].
However, DHT-induced cell proliferation in ER-positive MCF-7 breast cancer cells is inhibited by an ER-α antagonist, ICI 182,780, but not by the AR inhibitor flutamide [7]. DHT may interact with ERs to induce proliferation in ER-α positive breast cancer cells.
6. Integrin αvβ3 as a Receptor for Resveratrol
Resveratrol is a comprehensively studied, naturally occurring polyphenol with desirable properties in several biologic models. These activities include cardiovascular protection [44] and remarkable anti-cancer properties [45]. Whether resveratrol can have substantive clinical anticancer properties has repeatedly been subjected to question, because of the agent’s short half-life in the circulation of the intact organism and its rapid intracellular metabolism/turnover rate [46].
6.1. Resveratrol-Induced Apoptosis Signal Transduction Pathways: ERK1/2 and AMPK
A cell surface receptor for resveratrol on integrin αvβ3 has been identified by our group [47]. The existence of such a receptor suggests its ability to transduce the plasma and phosphorylated p53-dependent apoptosis. The signal transduction pathway involved in AMP-activated protein kinase (AMPK) activation was subsequently discovered to be associated with the action of resveratrol [48]. It is also remarkable that the receptors for steroid hormones and for resveratrol on the integrin do not appear to interact with one another. Both steroid hormones and resveratrol activate intracellular pools of extracellular signal-regulated kinases 1/2 (ERK1/2), but resveratrol is pro-apoptotic [49] and steroid hormones are anti-apoptotic by ERK1/2-dependent pathways [50]. The pro-apoptotic activity of resveratrol is blocked by Arg-Gly-Asp (RGD) peptides, which bind to the head of the extracellular domain of integrin αvβ3. This suggests that an important binding site for resveratrol may be proximal to the RGD peptide receptor on this integrin. However, recent evidence suggests that the cysteine-rich domain of the integrin may include the binding site for resveratrol.
The role of activated AMPK in cancer cell proliferation is controversial [51,52]. AMPK kinase (AMPKK) is responsive to activate AMPK by phosphorylation at Thr-172. The liver kinase B1 (LKB1) is a serine–threonine kinase that contributes to the regulation of cell energy metabolism, cell proliferation, and cell polarity [53,54]. Cytochrome P450-1A1 (CYP1A1) promotes breast cancer proliferation and survival through the suppression of AMPK signaling [52]. Compound C, an inhibitor of AMPK, promotes apoptotic cell death in various cancer cells; example cells include breast cancer cells and glioma [51]. A pharmacologic analogue of AMP—5-amino-1-β-Dffff-ribofuranosyl-imidazole-4-carboxamide (AICAR)—is an AMPK inhibitor with anticancer properties based upon activation of LKB1 [55]. However, it is not clear that activated AMPK is linked to resveratrol-induced apoptosis.
6.2. Resveratrol-Induced Nuclear COX-2
Resveratrol is able to induce the nuclear accumulation of cyclooxygenase-2 (COX-2) [45,49,56,57,58]. An index of tumor cell aggressiveness is build-up of cytoplasmic COX-2 [59,60], whose principal product is prostaglandins. COX-2 inhibition may improve clinical outcomes of certain cancers or be a cancer preventive, in the case of colon carcinoma [61].
Inducible accumulation of nuclear COX-2 is a wholly different biologic product. It is pro-apoptotic, and can interact with Ser-15-phosphorylated p53 and act as a co-activator [47,49,56,57,58]. ERK1/2 activation fosters the nuclear complexation of p53, p300, and COX-2 [49,56]. P300 is a co-activator of pro-apoptotic p53 [47,62], and also supports the accumulation of nuclear hormone receptors [63].
That the pro-apoptotic activity of resveratrol depends upon the nuclear accumulation of COX-2 suggests that pharmacologic COX-2 inhibitors, such as anti-inflammatory agents, also render them as inactivators of the anticancer (pro-apoptotic) activity of resveratrol and other polyphenols. NS-398 is an example of another experimental anti-inflammatory agent that also blocks the resveratrol-induced nuclear accumulation of COX-2 [49,56,57,58,64]. Concomitant administration of such agents with resveratrol may reduce pro-apoptotic activity of the stilbene in clinical applications.
6.3. Other Mechanisms Involved in Resveratrol-Induced Anti-Proliferation in Breast Cancers
Induced by resveratrol, apoptosis promoted in MCF-7 breast cancer cells by resveratrol depends upon the downregulation of anti-apoptotic Bcl-2. The mechanism of this downregulation is not clear, but may be linked to mitochondrial membrane actions of the stilbene that increase reactive oxygen species and nitric oxide production [65].
Nuclear factor κB (NF-κB), a regulator of Bcl-2 expression, and calpain protease activity, a regulator of NF-κB, are both inhibited by resveratrol [66,67]. NF-κB and calpain activities are PI-3K-dependent. NF-κB inhibition may result in diminished matrix metalloproteinase (MMP)-9 activity and decreased cell migration. Such observations suggest that resveratrol-induced apoptosis in MCF-7 cells could involve an oxidative, caspase-independent mechanism. The inhibitory effect of resveratrol is mediated in part through the suppression of activation of the PI-3K/Akt signaling pathway, whereby inhibition of PI-3K signaling converges with Bcl-2 through NF-κB and calpain protease activity [66]. Resveratrol also modulates the cell cycle and induces apoptosis in MCF-7 breast tumor cells by interfering with the ERα-dependent PI-3K pathway.
7. Interaction of Resveratrol and DHT
Both resveratrol and DHT activate ERK1/2. On the other hand, in breast cancer resveratrol inhibits PI-3K/AKT activation, which is involved in anti-apoptotic pathways and is negatively regulated by phosphatase and tensin homolog (PTEN). In addition, nuclear PTEN affects the cell cycle by negatively regulating the ERK pathway and cyclin D [68,69]. Interestingly, reduced PTEN protein levels are reported in sporadic breast cancers [29,70]. ER-α downregulates the accumulation of PTEN through PI-3K activation in breast cancer cells [71]. The level of PTEN protein in MCF-7 cells is significantly lower than that in MDA-MB 231 cells, and this is correlated with ER-α-positive status in MCF-7 cells. Resveratrol stimulates PTEN expression through AR inhibition [68]. Since DHT via ER-α stimulates ER-α-positive breast cancer proliferation, it is not surprising that DHT decreases PTEN expression and resveratrol increases PTEN expression in breast cancer cell lines, and thus inhibits proliferation [72].
In ER-negative human breast cancer cells, we have already demonstrated the susceptibility to DHT stimulation [7]. Resveratrol inhibits DHT-induced cell proliferation in MDA-MB cells. However, the inhibitory effect of resveratrol on DHT is not at the level of the receptor on integrin αvβ3, since ERK1/2 activation had an additive effect in the combination treatment of resveratrol and DHT. In both androgen-dependent and -independent prostate cancer cells, resveratrol inhibits AR transcriptional activity, but does not affect the total and nuclear AR levels [73]. Thus, the inhibitory effects of resveratrol on AR activity result from mechanisms other than AR nuclear translocation [74]. Resveratrol inhibits the binding of AR to the enhancer region of prostate-specific antigen (PSA) and decreases the acetylation of AR [75], although other studies suggest that resveratrol may not affect AR binding to DNA [74]. Resveratrol reduces the production of PSA, a notable target gene of AR. Resveratrol treatment also decreases the mRNA level of AR-regulated genes, such as NKX 3.1.
The interaction between steroid hormones and growth factors plays an important role in breast cancer development. DHT increases both epidermal growth factor receptor (EGFR) numbers and receptor–ligand affinity in androgen-sensitive prostate cancer cells; this correlates with increased EGF binding and an enhanced mitogenic response to EGF [76,77]. DHT up-regulates the levels of phosphorylation of EGFR (pEGFR) and its downstream proteins AKT (pAKT) and ERK1/2 (pERK) in AR-positive cells. However, the expression of EGFR in human breast cancer tissues has an inverse relationship with expression of the ER-α, and may be associated with a poor clinical outcome [78]. Thus, cross-talk between EGF and DHT may be more dominant in ER-negative than in ER-positive breast cancer cells. In addition to inhibiting DHT-induced signal transduction and biological activities, resveratrol is able to directly bind to EGFR and inhibit EGFR phosphorylation [74]. A summary of resveratrol- and DHT-affected signal transduction is listed in Table 2.
Table 2.
Effect of resveratrol and dihydrotestosterone (DHT) on the signal transduction pathway in cancer cells.
| Resveratrol | DHT | |
|---|---|---|
| Binding Site | Integrin αvβ3 | Integrin αvβ3/ER-α/AR |
| ERK1/2 | ↑ | ↑ |
| PI-3K | ↓ | ↑ |
| AKT | ↓ | ↑ |
| PTEN | ↑ | ↓ |
↑: increase, ↓: decrease.
8. Effect of DHT and Resveratrol on Metastasis
Metastasis is the primary cause of death in breast cancer patients. Cell migration and invasion play important roles in neoplastic metastasis. Cell proliferation, differentiation, apoptosis, and cell motility are prompted and controlled by a host of growth factors and hormones. The extracellular matrix (ECM) of human breast tumor cells has several effects of such cells: ECM is mitogenic for fibroblasts, and also stimulates the synthesis of collagen and elastin [79,80]. Both effects contribute to the desmoplastic response to human breast cancer in situ [79,80]. Maintenance of the differentiated state, including hormone and growth factor responsiveness, requires extracellular matrix proteins as a substrate for cells. The metastatic spread of cancer cells involves a complex process of detachment via anti-adhesion molecules and attachment and migration through adhesion [81]. In addition, DHT modulates the mechanoreception of human osteoblastic cells. DHT modulates the expression of adhesion molecules, such as fibronectin and the fibronectin receptor [82]. Some effects of DHT and resveratrol on receptors that play a role in metastasis are listed in Table 3.
Table 3.
Effects of resveratrol and DHT on the expression of receptors and activities in cancer cells.
| Resveratrol | DHT | |
|---|---|---|
| Integrin | β3 ↑ | α2β1 ↓ |
| EGFR | ↓ | ↑ |
| VEGFR | ─ | ↑ |
| VEGF | ↓ | ↑ |
↑: increase, ↓: decrease, ─: no effect.
9. Integrin αvβ3 and Metastasis
Integrin αvβ3 in the endothelial cell membrane is essential to the migration of capillaries into cancer tissue. This integrin is also a survival factor for endothelial cells [83]. The expression of integrin αvβ3 appears to play a key role in the development of bone metastasis from breast cancer [84]. Treatment with DHT downregulates the cell surface expression of integrin α2β1, but has little effect on the levels of integrin α3β1 and α5β1 in prostate cancer PC-3 cells containing transfected ARs [81]. Androgen also decreases the adhesion of AR-transfected PC-3 cells to collagen type I. Integrins αvβ3 and αvβ5 are critical components of the process of angiogenesis, and are a rationale for multiple attempts to base therapeutic anti-angiogenesis on integrin antagonists [85]. Downstream transduction of signals generated at surface αvβ3 importantly regulates VEGF expression in breast cancer [85]. Integrin αvβ3 clustering promotes the recruitment of p66Shc, and subsequently the phosphorylation of β3-associated p66Shc to upregulate VEGF expression. An important facet of mediation by integrin αvβ3 of VEGF expression and cancer-related angiogenesis is the phosphorylation of p66Shc [44]. In urinary bladder cancer patients, castration reduces tumor cell growth by DHT in vivo and decreases thrombospondin-1 (TSP1) expression [86]. Resveratrol has been shown to interact with the integrin β3 subunit, raising the possibility that inhibition of endothelial αvβ3 integrin function may contribute to the stilbene’s angiosuppressive activity [87]. Via ER-α, resveratrol increases the interaction between caveolin-1 (Cav-1) and c-Src, and increases the phosphorylation of Cav-1, c-Src, and eNOS in human umbilical vein ECs (HUVECs) [88]. In vivo, the angiogenesis of chick embryo area vasculosa and of mouse B16 melanoma are subject to inhibition by resveratrol. The polyphenol also blocks integrin-dependent vascularization models, such as αvβ3-linked endothelial wall adhesion and migration of integrin monomer β3 in focal adhesion contacts [87]. The latter may be relevant to management of ER-negative breast cancer.
10. VEGF and Metastasis
Integrin αvβ3-associated signaling regulates the growth of both prostate and breast tumors by influencing vascular endothelial growth factor (VEGF) expression [85]. Androgenic regulation of VEGF gene expression occurs shortly after androgen stimulation [89]. DHT importantly upregulates VEGF mRNA abundance [90], and VEGF biological activity is increased by DHT. Androgen regulates prostate blood flow, and VEGF is involved in blood flow regulation, with an activity equal to that of DHT.
Levels of the p66Shc protein are increased in cell lines with highly metastatic ability and in lymph node-positive tumors [91]. Downregulation of p66Shc inhibits VEGF expression, as well as tumor growth and angiogenesis in vivo [36]. Androgens have indirect effects on these cells via the upregulation of stromal VEGF production and angiogenesis. The use of VEGF inhibition as a substitute for anti-androgenic therapy may be effective against prostate diseases, especially disease that is relatively independent of androgens and that is hypervascular. VEGF-induced new blood vessel formation that is a function of reactive oxygen species (ROS)-dependent non-receptor protein tyrosine kinase (SRC kinase) activation is also inhibited by resveratrol [92].
11. Conclusions
The regulation of breast cancer cell proliferation is conventionally regarded as a function of the degree to which ER-positive cells have access to estrogen and to systemic polypeptide growth factors. Beyond surgery, tumor irradiation, and chemotherapy, the management of breast cancer emphasizes the long-term suppression of the action of endogenous estrogen with tamoxifen, or inhibition of estrogen synthesis with aromatase inhibition. Non-genomically, estrogen may support breast tumor growth through ER-like proteins in the membrane. This is now under investigation [93].
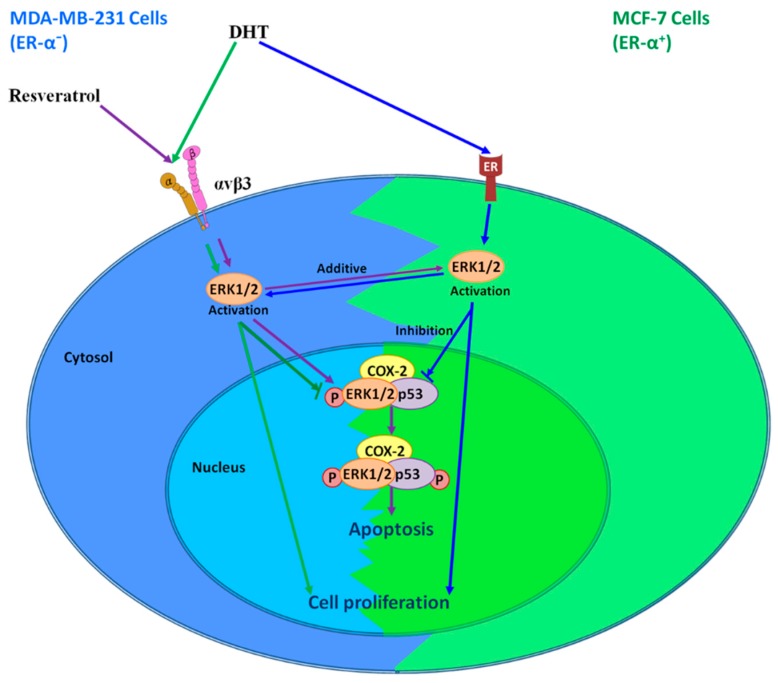
Identification of the resveratrol receptor site on integrin oteinon breast cancer cells and other solid tumor cells provides useful insights into the actions of this stilbene. Resveratrol binds to the integrin αvβ3 and rapidly activates ERK1/2 and AMPK to initiate the nuclear accumulation of COX-2 and p53-dependent apoptosis (Figure 1), regardless of uptake of the compound and chemical processing, which have been widely studied.
Figure 1.
Signal transduction pathways in the actions of resveratrol and DHT in different breast cancer cells. Extracellular signal-regulated kinases 1/2 (ERK1/2) activated at the plasma membrane are a result of resveratrol binding, and predictably result in cyclooxygenase-2 (COX-2) expression. Newly-generated COX-2 complexes with phosphorylated ERK1/2 (pERK1/2), is subject to SUMOylation, and then translocates to the nuclear compartment. In the nucleus, COX-2 and modified/activated p53 act as a transcription factor complex, causing the expression of p53-responsive genes. On the other hand, DHT binds to membrane estrogen receptor (ER)-α in ER-α-positive breast cancer cells, while it binds to integrin αvβ3 in ER-α-negative breast cancer cells. DHT activates ERK1/2 and induces cell proliferation. Activated ERK1/2 has discrete functions, depending upon whether activation in cancer cells is a response to resveratrol or DHT. ERK1/2 activation in response to resveratrol causes apoptosis. In contrast, DHT-activated ERK1/2 disrupts resveratrol-induced anti-proliferation. P: phosphorylation. ↓: active, ↓: inhibit.
DHT actins via integrin αvβ3 (Figure 1), behaving as a trophic agent for certain types of breast cancer cells [8]. Remarkably, the mechanisms involved in DHT-induced proliferative action differ between ER-α-negative and ER-α-positive cells in vitro. Receptors for DHT on integrin αvβ3 on the cell surface are required for the proliferative effect of the androgen in ER-negative cell; however, this may be irrelevant to ER-positive cells, whose cell surface ERs are required for the action of DHT. On the other hand, nuclear ARs may not play a role in the mechanism of DHT action in either type of cell [94]. What is somewhat surprising is that resveratrol-induced anti-proliferation is blocked by DHT, via a discrete receptor on the integrin. It is possible that the separate receptors for DHT and for resveratrol on the integrin can be modulated/inhibited to permit unimpeded expression of the anticancer actions of resveratrol at integrin αvβ3. It will also be useful to determine whether the resveratrol receptor on the integrin is related to the stilbene’s enhancement of breast cancer cell retention of doxorubicin [95], and whether this receptor mediates downregulation of DNA repair genes in tumor cells by resveratrol [96]. This is relevant to the radiosensitivity/radio-resistance of cancer cells. Currently, sulforaphane (SFN), epigallocatechin-3-gallate (EGCG), and other herb medicines have been shown to increase ER-α expression in ER-negative breast cancer MDA-MB cells [97,98]. However, resveratrol’s potential has not been examined yet. To understand the role of integrin αvβ3 in DHT and resveratrol-induced biologic activities in breast cancer should help with the clinical manipulation of breast cancers.
Acknowledgments
The authors appreciate Kelly Keating, Pharmaceutical Research Institute, Albany College of Pharmacy and Health Sciences, for her superior proofreading expertise.
Abbreviations
| ACTN4 | Actin-binding protein, actinin α 4 |
| AMPK | AMP-activated protein kinase |
| AMPKK | AMP-activated protein kinase kinase |
| 7α-APTADD | 7α (4′-amino) phenylthio-1,4-androstadiene-3,17-dione |
| AR | Androgen receptor |
| BRCA1 | Breast cancer 1 gene |
| Cav-1 | Caveolin-1 |
| COX-2 | Cyclooxygenase-2 |
| CYP1A1 | Cytochrome P450-1A1 |
| DHEAS | Dehydroepiandrosterone sulfate |
| DHT | Dihydrotestosterone |
| E1 | Estrone |
| E1S | Estrone sulfate |
| EGFR | Epidermal growth factor receptor |
| ER | Estrogen receptor |
| ERK1/2 | Extracellular signal-regulated kinases |
| FAK | Focal adhesion kinase |
| HER2 | Human epidermal growth factor receptor 2 |
| 17β-HSD1 | 17β-hydroxysteroid hydrogenase type 1 |
| iARs | Classical intracellular androgen receptors |
| LKB1 | Liver kinase B1 |
| MAPK | Mitogen-activated protein kinase |
| mARs | Membrane androgen receptors |
| MMP-2 | Matrix metalloproteinase-2 |
| PI-3K | Phosphatidylinositol-3-kinase |
| PKC δ | Protein kinase C δ |
| PR | Progesterone receptor |
| PTEN | Phosphatase and tensin homolog |
| RGD | Arg-Gly-Asp |
| TSP1 | Thrombospondin-1 |
| VEGF | Vascular endothelial growth factor |
Author Contributions
Organization, writing draft, revision, and submission, Y.H., H.-Y.L., Z.-L.L., Y.-R.C., and Y.-J.S.; review and editing. J.W.-P., K.W., and P.J.D.; visualization, Z.-L.L. and Y.-J.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research described in this article was supported in part by a research award from the Ta-Cheng Tung Foundation; from the Chair Professor Research Fund to K. Wang and to J. Whang-Peng; from the “TMU Research Center of Cancer Translational Medicine” from The Featured Areas Research Center Program, within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan; by grants from the Ministry of Science and Technology, Taiwan (MOST107-2314-B-038-017 and MOST108-2314-B-038-050); an endowment established by M. Frank Rudy and Marjorie Rudy; and a gift from Paul J. Davis to Albany College of Pharmacy and Health Sciences.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Levin E.R. Membrane oestrogen receptor alpha signalling to cell functions. J. Physiol. 2009;587:5019–5023. doi: 10.1113/jphysiol.2009.177097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shih Y.W., Chien S.T., Chen P.S., Lee J.H., Wu S.H., Yin L.T. Alpha-mangostin suppresses phorbol 12-myristate 13-acetate-induced MMP-2/MMP-9 expressions via alphavbeta3 integrin/FAK/ERK and NF-kappaB signaling pathway in human lung adenocarcinoma A549 cells. Cell Biochem. Biophys. 2010;58:31–44. doi: 10.1007/s12013-010-9091-2. [DOI] [PubMed] [Google Scholar]
- 3.Fujita M., Ieguchi K., Cedano-Prieto D.M., Fong A., Wilkerson C., Chen J.Q., Wu M., Lo S.H., Cheung A.T., Wilson M.D., et al. An integrin binding-defective mutant of insulin-like growth factor-1 (R36E/R37E IGF1) acts as a dominant-negative antagonist of the IGF1 receptor (IGF1R) and suppresses tumorigenesis but still binds to IGF1R. J. Biol. Chem. 2013;288:19593–19603. doi: 10.1074/jbc.M113.470872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapraeger A.C. Synstatin: A selective inhibitor of the syndecan-1-coupled IGF1R-alphavbeta3 integrin complex in tumorigenesis and angiogenesis. FEBS J. 2013;280:2207–2215. doi: 10.1111/febs.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giovannelli P., Di Donato M., Galasso G., Di Zazzo E., Medici N., Bilancio A., Migliaccio A., Castoria G. Breast cancer stem cells: The role of sex steroid receptors. World J. Stem Cells. 2019;11:594–603. doi: 10.4252/wjsc.v11.i9.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovannelli P., Di Donato M., Galasso G., Di Zazzo E., Bilancio A., Migliaccio A. The Androgen Receptor in Breast Cancer. Front. Endocrinol. 2018;9:492. doi: 10.3389/fendo.2018.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin H.Y., Sun M., Lin C., Tang H.Y., London D., Shih A., Davis F.B., Davis P.J. Androgen-induced human breast cancer cell proliferation is mediated by discrete mechanisms in estrogen receptor-alpha-positive and -negative breast cancer cells. J. Steroid Biochem. Mol. Biol. 2009;113:182–188. doi: 10.1016/j.jsbmb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Lin H.Y., Hsieh M.T., Cheng G.Y., Lai H.Y., Chin Y.T., Shih Y.J., Nana A.W., Lin S.Y., Yang Y.S.H., Tang H.Y., et al. Mechanisms of action of nonpeptide hormones on resveratrol-induced antiproliferation of cancer cells. Ann. N. Y. Acad. Sci. 2017;1403:92–100. doi: 10.1111/nyas.13423. [DOI] [PubMed] [Google Scholar]
- 9.Jang Y.G., Go R.E., Hwang K.A., Choi K.C. Resveratrol inhibits DHT-induced progression of prostate cancer cell line through interfering with the AR and CXCR4 pathway. J. Steroid Biochem. Mol. Biol. 2019;192:105406. doi: 10.1016/j.jsbmb.2019.105406. [DOI] [PubMed] [Google Scholar]
- 10.Chin Y.T., Yang S.H., Chang T.C., Changou C.A., Lai H.Y., Fu E., HuangFu W.C., Davis P.J., Lin H.Y., Liu L.F. Mechanisms of dihydrotestosterone action on resveratrol-induced anti-proliferation in breast cancer cells with different ERalpha status. Oncotarget. 2015;6:35866–35879. doi: 10.18632/oncotarget.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin H.Y., Chin Y.T., Yang Y.C., Lai H.Y., Wang-Peng J., Liu L.F., Tang H.Y., Davis P.J. Thyroid Hormone, Cancer, and Apoptosis. Compr. Physiol. 2016;6:1221–1237. doi: 10.1002/cphy.c150035. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y., Li Z., Shih Y., Davis P., Whang-Peng J., Lin H., Wang C.C. Thyroid hormone, PD-L1, and cancer. J. Cancer Res. Pract. 2019;6:162. [Google Scholar]
- 13.Davis P.J., Tang H.Y., Hercbergs A., Lin H.Y., Keating K.A., Mousa S.A. Bioactivity of Thyroid Hormone Analogs at Cancer Cells. Front. Endocrinol. 2018;9:739. doi: 10.3389/fendo.2018.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toth-Fejel S., Cheek J., Calhoun K., Muller P., Pommier R.F. Estrogen and androgen receptors as comediators of breast cancer cell proliferation: Providing a new therapeutic tool. Arch. Surg. 2004;139:50–54. doi: 10.1001/archsurg.139.1.50. [DOI] [PubMed] [Google Scholar]
- 15.Schover L.R. Androgen therapy for loss of desire in women: Is the benefit worth the breast cancer risk? Fertil. Steril. 2008;90:129–140. doi: 10.1016/j.fertnstert.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 16.Yeh S., Hu Y.C., Wang P.H., Xie C., Xu Q., Tsai M.Y., Dong Z., Wang R.S., Lee T.H., Chang C. Abnormal mammary gland development and growth retardation in female mice and MCF7 breast cancer cells lacking androgen receptor. J. Exp. Med. 2003;198:1899–1908. doi: 10.1084/jem.20031233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayer-Garner I.B., Smoller B. Androgen receptors: A marker to increase sensitivity for identifying breast cancer in skin metastasis of unknown primary site. Mod. Pathol. 2000;13:119–122. doi: 10.1038/modpathol.3880021. [DOI] [PubMed] [Google Scholar]
- 18.Agoff S.N., Swanson P.E., Linden H., Hawes S.E., Lawton T.J. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am. J. Clin. Pathol. 2003;120:725–731. doi: 10.1309/42F00D0DJD0J5EDT. [DOI] [PubMed] [Google Scholar]
- 19.Niemeier L.A., Dabbs D.J., Beriwal S., Striebel J.M., Bhargava R. Androgen receptor in breast cancer: Expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod. Pathol. 2010;23:205–212. doi: 10.1038/modpathol.2009.159. [DOI] [PubMed] [Google Scholar]
- 20.Pristauz G., Petru E., Stacher E., Geigl J.B., Schwarzbraun T., Tsybrovskyy O., Winter R., Moinfar F. Androgen receptor expression in breast cancer patients tested for BRCA1 and BRCA2 mutations. Histopathology. 2010;57:877–884. doi: 10.1111/j.1365-2559.2010.03724.x. [DOI] [PubMed] [Google Scholar]
- 21.Henderson B.E., Feigelson H.S. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 22.Peng Y., Li Y., Gellert L.L., Zou X., Wang J., Singh B., Xu R., Chiriboga L., Daniels G., Pan R., et al. Androgen receptor coactivator p44/Mep50 in breast cancer growth and invasion. J. Cell Mol. Med. 2010;14:2780–2789. doi: 10.1111/j.1582-4934.2009.00936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ligr M., Patwa R.R., Daniels G., Pan L., Wu X., Li Y., Tian L., Wang Z., Xu R., Wu J., et al. Expression and function of androgen receptor coactivator p44/Mep50/WDR77 in ovarian cancer. PLoS ONE. 2011;6:e26250. doi: 10.1371/annotation/fe4fca93-8211-430e-bfe0-8a371b9cc20d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khurana S., Chakraborty S., Cheng X., Su Y.T., Kao H.Y. The actin-binding protein, actinin alpha 4 (ACTN4), is a nuclear receptor coactivator that promotes proliferation of MCF-7 breast cancer cells. J. Biol. Chem. 2011;286:1850–1859. doi: 10.1074/jbc.M110.162107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jasavala R., Martinez H., Thumar J., Andaya A., Gingras A.C., Eng J.K., Aebersold R., Han D.K., Wright M.E. Identification of putative androgen receptor interaction protein modules: Cytoskeleton and endosomes modulate androgen receptor signaling in prostate cancer cells. Mol. Cell Proteomics. 2007;6:252–271. doi: 10.1074/mcp.M600169-MCP200. [DOI] [PubMed] [Google Scholar]
- 26.Gu S., Papadopoulou N., Gehring E.M., Nasir O., Dimas K., Bhavsar S.K., Foller M., Alevizopoulos K., Lang F., Stournaras C. Functional membrane androgen receptors in colon tumors trigger pro-apoptotic responses in vitro and reduce drastically tumor incidence in vivo. Mol. Cancer. 2009;8:114. doi: 10.1186/1476-4598-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papadopoulou N., Papakonstanti E.A., Kallergi G., Alevizopoulos K., Stournaras C. Membrane androgen receptor activation in prostate and breast tumor cells: Molecular signaling and clinical impact. IUBMB Life. 2009;61:56–61. doi: 10.1002/iub.150. [DOI] [PubMed] [Google Scholar]
- 28.Kampa M., Theodoropoulou K., Mavromati F., Pelekanou V., Notas G., Lagoudaki E.D., Nifli A.P., Morel-Salmi C., Stathopoulos E.N., Vercauteren J., et al. Novel oligomeric proanthocyanidin derivatives interact with membrane androgen sites and induce regression of hormone-independent prostate cancer. J. Pharmacol. Exp. Ther. 2011;337:24–32. doi: 10.1124/jpet.110.177246. [DOI] [PubMed] [Google Scholar]
- 29.Pelekanou V., Notas G., Sanidas E., Tsapis A., Castanas E., Kampa M. Testosterone membrane-initiated action in breast cancer cells: Interaction with the androgen signaling pathway and EPOR. Mol. Oncol. 2010;4:135–149. doi: 10.1016/j.molonc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinn A.L., Burak W.E., Jr., Brueggemeier R.W. Effects of matrix components on aromatase activity in breast stromal cells in culture. J. Steroid Biochem. Mol. Biol. 1999;70:249–256. doi: 10.1016/S0960-0760(99)00113-2. [DOI] [PubMed] [Google Scholar]
- 31.Birrell S.N., Bentel J.M., Hickey T.E., Ricciardelli C., Weger M.A., Horsfall D.J., Tilley W.D. Androgens induce divergent proliferative responses in human breast cancer cell lines. J. Steroid Biochem. Mol. Biol. 1995;52:459–467. doi: 10.1016/0960-0760(95)00005-K. [DOI] [PubMed] [Google Scholar]
- 32.Szelei J., Jimenez J., Soto A.M., Luizzi M.F., Sonnenschein C. Androgen-induced inhibition of proliferation in human breast cancer MCF7 cells transfected with androgen receptor. Endocrinology. 1997;138:1406–1412. doi: 10.1210/endo.138.4.5047. [DOI] [PubMed] [Google Scholar]
- 33.Greeve M.A., Allan R.K., Harvey J.M., Bentel J.M. Inhibition of MCF-7 breast cancer cell proliferation by 5alpha-dihydrotestosterone; a role for p21(Cip1/Waf1) J. Mol. Endocrinol. 2004;32:793–810. doi: 10.1677/jme.0.0320793. [DOI] [PubMed] [Google Scholar]
- 34.Kallergi G., Mavroudis D., Georgoulias V., Stournaras C. Phosphorylation of FAK, PI-3K, and impaired actin organization in CK-positive micrometastatic breast cancer cells. Mol. Med. 2007;13:79–88. doi: 10.2119/2006-00083.Kallergi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hecker T.P., Grammer J.R., Gillespie G.Y., Stewart J., Jr., Gladson C.L. Focal adhesion kinase enhances signaling through the Shc/extracellular signal-regulated kinase pathway in anaplastic astrocytoma tumor biopsy samples. Cancer Res. 2002;62:2699–2707. [PubMed] [Google Scholar]
- 36.De S., Razorenova O., McCabe N.P., O’Toole T., Qin J., Byzova T.V. VEGF-integrin interplay controls tumor growth and vascularization. Proc. Natl. Acad. Sci. USA. 2005;102:7589–7594. doi: 10.1073/pnas.0502935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajendran M., Thomes P., Zhang L., Veeramani S., Lin M.F. p66Shc—A longevity redox protein in human prostate cancer progression and metastasis: p66Shc in cancer progression and metastasis. Cancer Metastasis Rev. 2010;29:207–222. doi: 10.1007/s10555-010-9213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veeramani S., Yuan T.C., Lin F.F., Lin M.F. Mitochondrial redox signaling by p66Shc is involved in regulating androgenic growth stimulation of human prostate cancer cells. Oncogene. 2008;27:5057–5068. doi: 10.1038/onc.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S., Kumar S., Rajendran M., Alam S.M., Lin F.F., Cheng P.W., Lin M.F. Steroids up-regulate p66Shc longevity protein in growth regulation by inhibiting its ubiquitination. PLoS ONE. 2011;6:e15942. doi: 10.1371/journal.pone.0015942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonne-Hansen K., Lykkesfeldt A.E. Endogenous aromatization of testosterone results in growth stimulation of the human MCF-7 breast cancer cell line. J. Steroid Biochem. Mol. Biol. 2005;93:25–34. doi: 10.1016/j.jsbmb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Nativelle-Serpentini C., Lambard S., Seralini G.E., Sourdaine P. Aromatase and breast cancer: W39R, an inactive protein. Eur. J. Endocrinol. 2002;146:583–589. doi: 10.1530/eje.0.1460583. [DOI] [PubMed] [Google Scholar]
- 42.Burak W.E., Jr., Quinn A.L., Farrar W.B., Brueggemeier R.W. Androgens influence estrogen-induced responses in human breast carcinoma cells through cytochrome P450 aromatase. Breast Cancer Res. Treat. 1997;44:57–64. doi: 10.1023/A:1005782311558. [DOI] [PubMed] [Google Scholar]
- 43.Aka J.A., Mazumdar M., Chen C.Q., Poirier D., Lin S.X. 17beta-hydroxysteroid dehydrogenase type 1 stimulates breast cancer by dihydrotestosterone inactivation in addition to estradiol production. Mol. Endocrinol. 2010;24:832–845. doi: 10.1210/me.2009-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das S., Mitrovsky G., Vasanthi H.R., Das D.K. Antiaging properties of a grape-derived antioxidant are regulated by mitochondrial balance of fusion and fission leading to mitophagy triggered by a signaling network of Sirt1-Sirt3-Foxo3-PINK1-PARKIN. Oxid. Med. Cell Longev. 2014;2014:345105. doi: 10.1155/2014/345105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Lin H.Y., Davis P.J., Tang H.Y., Mousa S.A., Luidens M.K., Hercbergs A.H., Davis F.B. The pro-apoptotic action of stilbene-induced COX-2 in cancer cells: Convergence with the anti-apoptotic effect of thyroid hormone. Cell Cycle. 2009;8:1877–1882. doi: 10.4161/cc.8.12.8747. [DOI] [PubMed] [Google Scholar]
- 46.Patel K.R., Scott E., Brown V.A., Gescher A.J., Steward W.P., Brown K. Clinical trials of resveratrol. Ann. N. Y. Acad. Sci. 2011;1215:161–169. doi: 10.1111/j.1749-6632.2010.05853.x. [DOI] [PubMed] [Google Scholar]
- 47.Lin H.Y., Lansing L., Merillon J.M., Davis F.B., Tang H.Y., Shih A., Vitrac X., Krisa S., Keating T., Cao H.J., et al. Integrin alphaVbeta3 contains a receptor site for resveratrol. FASEB J. 2006;20:1742–1744. doi: 10.1096/fj.06-5743fje. [DOI] [PubMed] [Google Scholar]
- 48.Lin J.N., Lin V.C., Rau K.M., Shieh P.C., Kuo D.H., Shieh J.C., Chen W.J., Tsai S.C., Way T.D. Resveratrol modulates tumor cell proliferation and protein translation via SIRT1-dependent AMPK activation. J. Agric. Food Chem. 2010;58:1584–1592. doi: 10.1021/jf9035782. [DOI] [PubMed] [Google Scholar]
- 49.Lin H.Y., Tang H.Y., Keating T., Wu Y.H., Shih A., Hammond D., Sun M., Hercbergs A., Davis F.B., Davis P.J. Resveratrol is pro-apoptotic and thyroid hormone is anti-apoptotic in glioma cells: Both actions are integrin and ERK mediated. Carcinogenesis. 2008;29:62–69. doi: 10.1093/carcin/bgm239. [DOI] [PubMed] [Google Scholar]
- 50.Lin H.Y., Tang H.Y., Shih A., Keating T., Cao G., Davis P.J., Davis F.B. Thyroid hormone is a MAPK-dependent growth factor for thyroid cancer cells and is anti-apoptotic. Steroids. 2007;72:180–187. doi: 10.1016/j.steroids.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 51.Huang S.W., Wu C.Y., Wang Y.T., Kao J.K., Lin C.C., Chang C.C., Mu S.W., Chen Y.Y., Chiu H.W., Chang C.H., et al. p53 modulates the AMPK inhibitor compound C induced apoptosis in human skin cancer cells. Toxicol. Appl. Pharmacol. 2013;267:113–124. doi: 10.1016/j.taap.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez M., Potter D.A. CYP1A1 regulates breast cancer proliferation and survival. Mol. Cancer Res. 2013;11:780–792. doi: 10.1158/1541-7786.MCR-12-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaw R.J., Kosmatka M., Bardeesy N., Hurley R.L., Witters L.A., DePinho R.A., Cantley L.C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurst D., Taylor E.B., Cline T.D., Greenwood L.J., Compton C.L., Lamb J.D., Winder W.W. AMP-activated protein kinase kinase activity and phosphorylation of AMP-activated protein kinase in contracting muscle of sedentary and endurance-trained rats. Am. J. Physiol. Endocrinol. Metab. 2005;289:E710–E715. doi: 10.1152/ajpendo.00155.2005. [DOI] [PubMed] [Google Scholar]
- 55.Sun Y., Connors K.E., Yang D.Q. AICAR induces phosphorylation of AMPK in an ATM-dependent, LKB1-independent manner. Mol. Cell Biochem. 2007;306:239–245. doi: 10.1007/s11010-007-9575-6. [DOI] [PubMed] [Google Scholar]
- 56.Lin H.Y., Sun M., Tang H.Y., Simone T.M., Wu Y.H., Grandis J.R., Cao H.J., Davis P.J., Davis F.B. Resveratrol causes COX-2- and p53-dependent apoptosis in head and neck squamous cell cancer cells. J. Cell Biochem. 2008;104:2131–2142. doi: 10.1002/jcb.21772. [DOI] [PubMed] [Google Scholar]
- 57.Lin C., Crawford D.R., Lin S., Hwang J., Sebuyira A., Meng R., Westfall J.E., Tang H.Y., Lin S., Yu P.Y., et al. Inducible COX-2-dependent apoptosis in human ovarian cancer cells. Carcinogenesis. 2011;32:19–26. doi: 10.1093/carcin/bgq212. [DOI] [PubMed] [Google Scholar]
- 58.Lin H.Y., Delmas D., Vang O., Hsieh T.C., Lin S., Cheng G.Y., Chiang H.L., Chen C.E., Tang H.Y., Crawford D.R., et al. Mechanisms of ceramide-induced COX-2-dependent apoptosis in human ovarian cancer OVCAR-3 cells partially overlapped with resveratrol. J. Cell Biochem. 2013;114:1940–1954. doi: 10.1002/jcb.24539. [DOI] [PubMed] [Google Scholar]
- 59.Schmitz K.J., Callies R., Wohlschlaeger J., Kimmig R., Otterbach F., Bohr J., Lee H.S., Takeda A., Schmid K.W., Baba H.A. Overexpression of cyclo-oxygenase-2 is an independent predictor of unfavourable outcome in node-negative breast cancer, but is not associated with protein kinase B (Akt) and mitogen-activated protein kinase (ERK1/2, p38) activation or with Her-2/neu signalling pathways. J. Clin. Pathol. 2006;59:685–691. doi: 10.1136/jcp.2005.030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perdiki M., Korkolopoulou P., Thymara I., Agrogiannis G., Piperi C., Boviatsis E., Kotsiakis X., Angelidakis D., Diamantopoulou K., Thomas-Tsagli E., et al. Cyclooxygenase-2 expression in astrocytomas. Relationship with microvascular parameters, angiogenic factors expression and survival. Mol. Cell Biochem. 2007;295:75–83. doi: 10.1007/s11010-006-9275-7. [DOI] [PubMed] [Google Scholar]
- 61.Harris R.E. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. 2009;17:55–67. doi: 10.1007/s10787-009-8049-8. [DOI] [PubMed] [Google Scholar]
- 62.Song L., Gao M., Dong W., Hu M., Li J., Shi X., Hao Y., Li Y., Huang C. p85alpha mediates p53 K370 acetylation by p300 and regulates its promoter-specific transactivity in the cellular UVB response. Oncogene. 2011;30:1360–1371. doi: 10.1038/onc.2010.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalkhoven E. CBP and p300: HATs for different occasions. Biochem. Pharmacol. 2004;68:1145–1155. doi: 10.1016/j.bcp.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 64.Tang H.Y., Shih A., Cao H.J., Davis F.B., Davis P.J., Lin H.Y. Resveratrol-induced cyclooxygenase-2 facilitates p53-dependent apoptosis in human breast cancer cells. Mol. Cancer Ther. 2006;5:2034–2042. doi: 10.1158/1535-7163.MCT-06-0216. [DOI] [PubMed] [Google Scholar]
- 65.Quincozes-Santos A., Bobermin L.D., Latini A., Wajner M., Souza D.O., Goncalves C.A., Gottfried C. Resveratrol protects C6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1. PLoS ONE. 2013;8:e64372. doi: 10.1371/journal.pone.0064372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pozo-Guisado E., Merino J.M., Mulero-Navarro S., Lorenzo-Benayas M.J., Centeno F., Alvarez-Barrientos A., Fernandez-Salguero P.M. Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-kappaB. Int. J. Cancer. 2005;115:74–84. doi: 10.1002/ijc.20856. [DOI] [PubMed] [Google Scholar]
- 67.Sareen D., Darjatmoko S.R., Albert D.M., Polans A.S. Mitochondria, calcium, and calpain are key mediators of resveratrol-induced apoptosis in breast cancer. Mol. Pharmacol. 2007;72:1466–1475. doi: 10.1124/mol.107.039040. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y., Romigh T., He X., Orloff M.S., Silverman R.H., Heston W.D., Eng C. Resveratrol regulates the PTEN/AKT pathway through androgen receptor-dependent and -independent mechanisms in prostate cancer cell lines. Hum. Mol. Genet. 2010;19:4319–4329. doi: 10.1093/hmg/ddq354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steelman L.S., Chappell W.H., Abrams S.L., Kempf R.C., Long J., Laidler P., Mijatovic S., Maksimovic-Ivanic D., Stivala F., Mazzarino M.C., et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging. 2011;3:192–222. doi: 10.18632/aging.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bose S., Crane A., Hibshoosh H., Mansukhani M., Sandweis L., Parsons R. Reduced expression of PTEN correlates with breast cancer progression. Hum. Pathol. 2002;33:405–409. doi: 10.1053/hupa.2002.124721. [DOI] [PubMed] [Google Scholar]
- 71.Kappes H., Goemann C., Bamberger A.M., Loning T., Milde-Langosch K. PTEN expression in breast and endometrial cancer: Correlations with steroid hormone receptor status. Pathobiology. 2001;69:136–142. doi: 10.1159/000048768. [DOI] [PubMed] [Google Scholar]
- 72.Waite K.A., Sinden M.R., Eng C. Phytoestrogen exposure elevates PTEN levels. Hum. Mol. Genet. 2005;14:1457–1463. doi: 10.1093/hmg/ddi155. [DOI] [PubMed] [Google Scholar]
- 73.Harada N., Atarashi K., Murata Y., Yamaji R., Nakano Y., Inui H. Inhibitory mechanisms of the transcriptional activity of androgen receptor by resveratrol: Implication of DNA binding and acetylation of the receptor. J. Steroid Biochem. Mol. Biol. 2011;123:65–70. doi: 10.1016/j.jsbmb.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 74.Shi W.F., Leong M., Cho E., Farrell J., Chen H.C., Tian J., Zhang D. Repressive effects of resveratrol on androgen receptor transcriptional activity. PLoS ONE. 2009;4:e7398. doi: 10.1371/journal.pone.0007398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park S.Y., Lee Y.H., Choi K.C., Seong A.R., Choi H.K., Lee O.H., Hwang H.J., Yoon H.G. Grape seed extract regulates androgen receptor-mediated transcription in prostate cancer cells through potent anti-histone acetyltransferase activity. J. Med. Food. 2011;14:9–16. doi: 10.1089/jmf.2010.1264. [DOI] [PubMed] [Google Scholar]
- 76.Brass A.L., Barnard J., Patai B.L., Salvi D., Rukstalis D.B. Androgen up-regulates epidermal growth factor receptor expression and binding affinity in PC3 cell lines expressing the human androgen receptor. Cancer Res. 1995;55:3197–3203. [PubMed] [Google Scholar]
- 77.Zheng Y., Izumi K., Yao J.L., Miyamoto H. Dihydrotestosterone upregulates the expression of epidermal growth factor receptor and ERBB2 in androgen receptor-positive bladder cancer cells. Endocr. Relat. Cancer. 2011;18:451–464. doi: 10.1530/ERC-11-0010. [DOI] [PubMed] [Google Scholar]
- 78.Davidson N.E., Gelmann E.P., Lippman M.E., Dickson R.B. Epidermal growth factor receptor gene expression in estrogen receptor-positive and negative human breast cancer cell lines. Mol. Endocrinol. 1987;1:216–223. doi: 10.1210/mend-1-3-216. [DOI] [PubMed] [Google Scholar]
- 79.Kao R.T., Hall J., Stern R. Collagen and elastin synthesis in human stroma and breast carcinoma cell lines: Modulation by the extracellular matrix. Connect. Tissue Res. 1986;14:245–255. doi: 10.3109/03008208609017468. [DOI] [PubMed] [Google Scholar]
- 80.Shekhar M.P., Werdell J., Santner S.J., Pauley R.J., Tait L. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: Implications for tumor development and progression. Cancer Res. 2001;61:1320–1326. [PubMed] [Google Scholar]
- 81.Evangelou A., Letarte M., Marks A., Brown T.J. Androgen modulation of adhesion and antiadhesion molecules in PC-3 prostate cancer cells expressing androgen receptor. Endocrinology. 2002;143:3897–3904. doi: 10.1210/en.2002-220156. [DOI] [PubMed] [Google Scholar]
- 82.Liegibel U.M., Sommer U., Tomakidi P., Hilscher U., Van Den Heuvel L., Pirzer R., Hillmeier J., Nawroth P., Kasperk C. Concerted action of androgens and mechanical strain shifts bone metabolism from high turnover into an osteoanabolic mode. J. Exp. Med. 2002;196:1387–1392. doi: 10.1084/jem.20021017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Engebraaten O., Trikha M., Juell S., Garman-Vik S., Fodstad O. Inhibition of in vivo tumour growth by the blocking of host alpha(v)beta3 and alphaII(b)beta3 integrins. Anticancer Res. 2009;29:131–137. [PubMed] [Google Scholar]
- 84.Takayama S., Ishii S., Ikeda T., Masamura S., Doi M., Kitajima M. The relationship between bone metastasis from human breast cancer and integrin alpha(v)beta3 expression. Anticancer Res. 2005;25:79–83. [PubMed] [Google Scholar]
- 85.Weis S.M., Cheresh D.A. alphaV integrins in angiogenesis and cancer. Cold Spring Harb. Perspect. Med. 2011;1:a006478. doi: 10.1101/cshperspect.a006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnson A.M., O’Connell M.J., Miyamoto H., Huang J., Yao J.L., Messing E.M., Reeder J.E. Androgenic dependence of exophytic tumor growth in a transgenic mouse model of bladder cancer: A role for thrombospondin-1. BMC Urol. 2008;8:7. doi: 10.1186/1471-2490-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Belleri M., Ribatti D., Savio M., Stivala L.A., Forti L., Tanghetti E., Alessi P., Coltrini D., Bugatti A., Mitola S., et al. alphavbeta3 Integrin-dependent antiangiogenic activity of resveratrol stereoisomers. Mol. Cancer Ther. 2008;7:3761–3770. doi: 10.1158/1535-7163.MCT-07-2351. [DOI] [PubMed] [Google Scholar]
- 88.Klinge C.M., Wickramasinghe N.S., Ivanova M.M., Dougherty S.M. Resveratrol stimulates nitric oxide production by increasing estrogen receptor alpha-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008;22:2185–2197. doi: 10.1096/fj.07-103366. [DOI] [PubMed] [Google Scholar]
- 89.Shibata Y., Kashiwagi B., Arai S., Fukabori Y., Suzuki K., Honma S., Yamanaka H. Direct regulation of prostate blood flow by vascular endothelial growth factor and its participation in the androgenic regulation of prostate blood flow in vivo. Endocrinology. 2004;145:4507–4512. doi: 10.1210/en.2004-0288. [DOI] [PubMed] [Google Scholar]
- 90.Sordello S., Bertrand N., Plouet J. Vascular endothelial growth factor is up-regulated in vitro and in vivo by androgens. Biochem. Biophys. Res. Commun. 1998;251:287–290. doi: 10.1006/bbrc.1998.9328. [DOI] [PubMed] [Google Scholar]
- 91.Jackson J.G., Yoneda T., Clark G.M., Yee D. Elevated levels of p66 Shc are found in breast cancer cell lines and primary tumors with high metastatic potential. Clin. Cancer Res. 2000;6:1135–1139. [PubMed] [Google Scholar]
- 92.Lin M.T., Yen M.L., Lin C.Y., Kuo M.L. Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol. Pharmacol. 2003;64:1029–1036. doi: 10.1124/mol.64.5.1029. [DOI] [PubMed] [Google Scholar]
- 93.Gu Y., Chen T., Lopez E., Wu W., Wang X., Cao J., Teng L. The therapeutic target of estrogen receptor-alpha36 in estrogen-dependent tumors. J. Transl. Med. 2014;12:16. doi: 10.1186/1479-5876-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin H.-Y., Davis F.B., Luidens M.K., Hercbergs A., Mousa S.A., Bharali D.J., Davis P.J. Newly-Recognized Small Molecule Receptors on Human Breast Cancer Cell Integrin αvβ3 that Affect Tumor Cell Behavior, Targeting New Pathways and Cell Death in Breast Cancer. InTech; Rijeka, Croatia: 2012. [(accessed on 18 April 2020)]. Available online: https://www.intechopen.com/books/targeting-new-pathways-and-cell-death-in-breast-cancer/newly-recognized-small-molecule-receptors-on-human-breast-cancer-cell-integrin-alphavbeta3-that-affe. [Google Scholar]
- 95.Kim T.H., Shin Y.J., Won A.J., Lee B.M., Choi W.S., Jung J.H., Chung H.Y., Kim H.S. Resveratrol enhances chemosensitivity of doxorubicin in multidrug-resistant human breast cancer cells via increased cellular influx of doxorubicin. Biochim. Biophys. Acta. 2014;1840:615–625. doi: 10.1016/j.bbagen.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 96.Leon-Galicia I., Diaz-Chavez J., Garcia-Villa E., Uribe-Figueroa L., Hidalgo-Miranda A., Herrera L.A., Alvarez-Rios E., Garcia-Mena J., Gariglio P. Resveratrol induces downregulation of DNA repair genes in MCF-7 human breast cancer cells. Eur. J. Cancer Prev. 2013;22:11–20. doi: 10.1097/CEJ.0b013e328353edcb. [DOI] [PubMed] [Google Scholar]
- 97.Gianfredi V., Nucci D., Vannini S., Villarini M., Moretti M. In vitro Biological Effects of Sulforaphane (SFN), Epigallocatechin-3-gallate (EGCG), and Curcumin on Breast Cancer Cells: A Systematic Review of the Literature. Nutr. Cancer. 2017;69:969–978. doi: 10.1080/01635581.2017.1359322. [DOI] [PubMed] [Google Scholar]
- 98.Gianfredi V., Vannini S., Moretti M., Villarini M., Bragazzi N.L., Izzotti A., Nucci D. Sulforaphane and Epigallocatechin Gallate Restore Estrogen Receptor Expression by Modulating Epigenetic Events in the Breast Cancer Cell Line MDA-MB-231: A Systematic Review and Meta-Analysis. J. Nutrigenet. Nutrigenom. 2017;10:126–135. doi: 10.1159/000480636. [DOI] [PubMed] [Google Scholar]