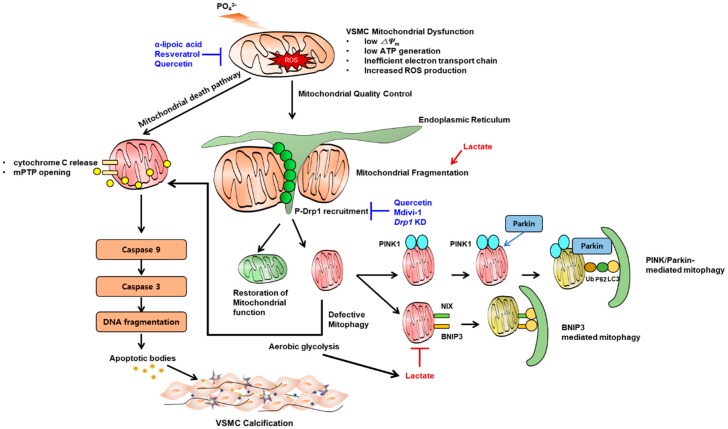
Figure 3.
Key mechanism by which mitochondrial dysfunction contributes to vascular calcification. High phosphate induces vascular smooth muscle cell (VSMC) mitochondrial dysfunction represented by low mitochondrial membrane potential, low ATP synthesis and increased reactive oxygen species as a result of inefficient electron transport chain. This leads to release of mitochondrial permeability transition pore (mPTP) and release of cytochrome C which in turn, induces a subsequent cascade of caspase-9 and caspase-3 activation and apoptosis. Mitochondria require quality control in the presence of mitochondrial stress. The representative phenomenon is mitochondrial fission which is mainly mediated by phosphorylated Drp1 recruitment on the site of fission. Once fragmented, dysfunctional mitochondria undergo mitophagy (canonical PINK1-parkin mediated or BCL2-Interacting Protein 3 (BNIP3) mediated). When mitophagic clearance is defective, dysfunctional mitochondria undergo apoptosis. Dysfunctional mitochondria tend to rely on aereobic glycolysis rather than oxidative phosphorylation which might further produce lactate. Lactate promotes mitochondrial fission and blocks mitophagy, both of which promote apoptosis. Some chemicals are preventive in experimental models of vascular calcification (shown in blue text, also see main text).