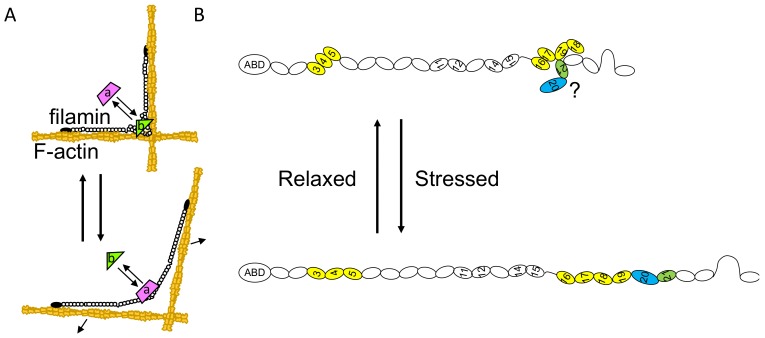
Figure 5.
A model of how mechanical forces regulate FLNC-partner interaction. (A) Contractile force of actomyosin or deformation of actin networks induces conformational changes of filamin molecule. Some binding partner (a, e.g., beta-integrin) interacts with exposed binding site under mechanical stress, whereas some (b, e.g., FilGAP for FLNA. FilGAP does not interact with FLNC [37]) dissociates when filamin molecule is deformed. (B) Predicted structure of FLNC subunit. Only one subunit is shown. The hinge-1 is spliced out during myogenesis but strand A of R16 is free from the folded domain, thereby the link between R15 and R16 possesses some flexibility. Colored domain pairs can potentially be dissociated by mechanical force. Because of unique insertion of 82 amino acids in R20 (blue), it is not known if R20 pairs with R21(green) to create cryptic binding site (Figure 2C). Domain pairs of R11-R12 and R14-R15 were suggested by small-angle X-ray scattering analysis but appears to be loose.