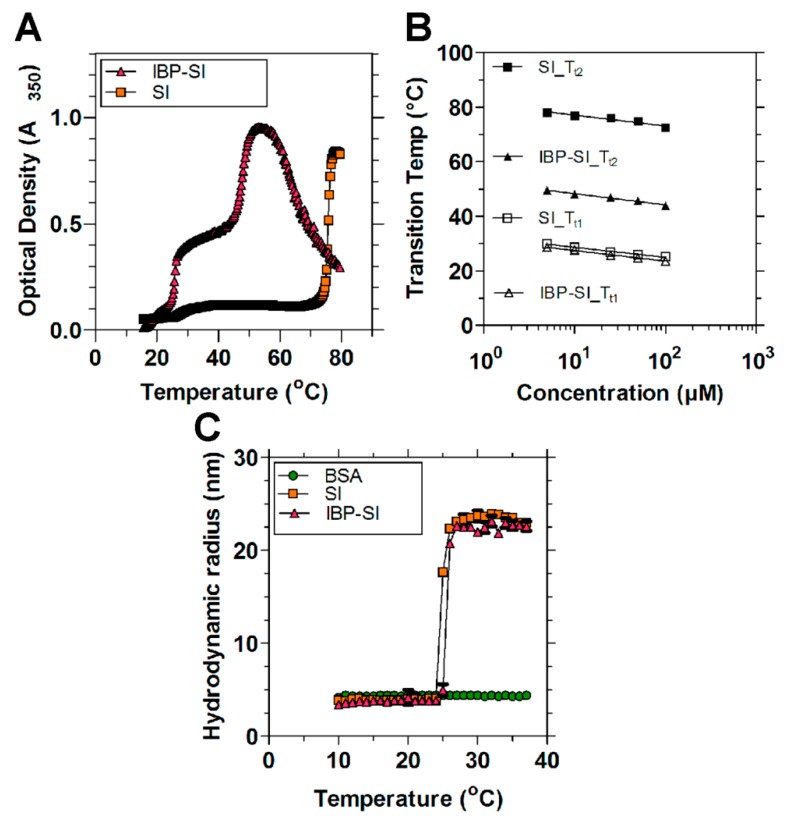
Figure 2.
ELP protein–polymers with or without ICAM-1-targeting peptides form nanoparticles at physiological temperature. (A) Temperature-induced assembly of ELPs was determined by measurement of optical density (OD350) as a function of temperature and concentration. Both SI and IBP-SI (25 μM in PBS) have two obvious inflections. Tt1 is consistent with the assembly of the hydrophobic I48, whereas Tt2 is associated with the bulk phase separation of hydrophilic S48. Diblock ELPs assemble into nanoparticles between Tt1 and Tt2. At this concentration, SI assembles into nanoparticles between 25.5 °C and 73.7 °C and IBP-SI assembles nanoparticles between 25.7 °C and 46.9 °C. (B) The concentration–temperature phase diagrams for SI and IBP-SI follow a log-linear relationship. The Tts of each ELP were fitted to the equation Tt = m Log10 (CELP) + b, where m is the slope, CELP is the concentration, and b is the transition temperature at 1 μM (Appendix A Table A1). (C) DLS was used to measure the self-assembly and hydrodynamic radius of ELP nanoparticles (25 μM in PBS) up to 37 °C. The ICAM-1 binding peptide has minimal influence on the assembly and radius of ELP nanoparticles. Bovine serum albumin (BSA) was used as an internal control. At 37 °C, SI and IBP-SI form nanoparticles with a hydrodynamic radius of 23.6 ± 0.4 nm and 21.9 ± 0.6 nm, respectively. Data are presented as mean ± SD.