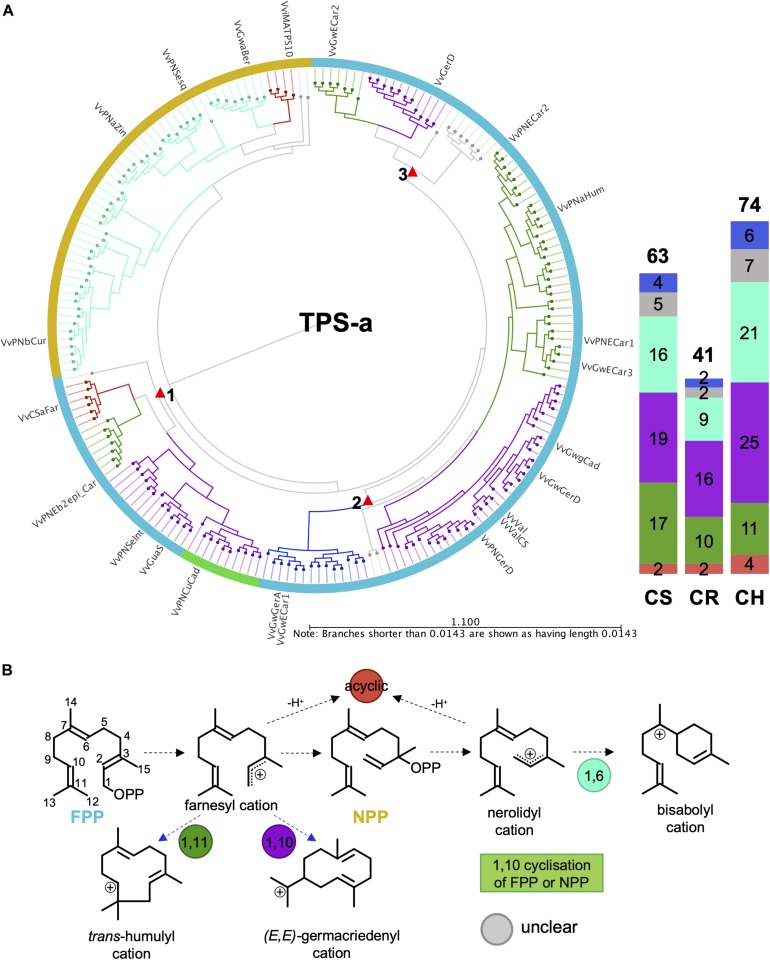
FIGURE 5.
(A) Active site phylogeny of putative VviTPS-a proteins from the diploid genomes and functionally characterized enzymes (outer labels) were used to predict the initial substrate (outer colored ring) and cyclization mechanism (branch color). The red triangles indicate subclades for enzymes that utilize farnesyl diphosphate (FPP) as initial substrate, shown by the blue outer ring. The yellow outer ring represents enzymes that utilize nerolidyl diphosphate (NPP) as initial substrate. Both FPP and NPP can be ionized or protonated to form the initial carbocation intermediates indicated in (B). Branches in (A) are colored according to these initial carbocation cyclization mechanisms and the subsequent carbocation formed, shown in (B). Deprotonation of the initial carbocation result in the formation of acyclic sesquiterpenes, as shown in (B).