Figure 3. Active domain motions in the glutamate transporter GltPh and the light-driven proton pump bR.
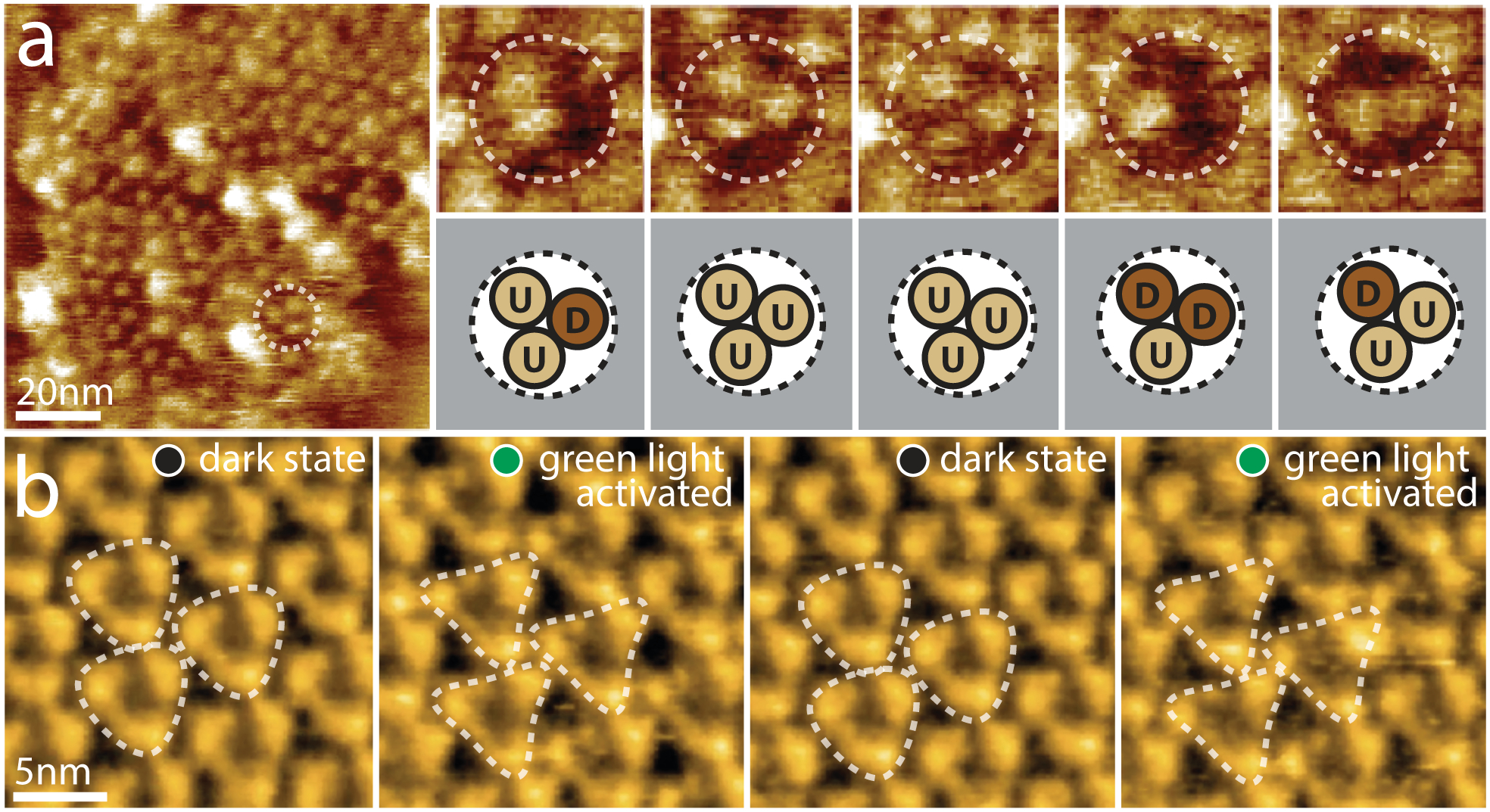
(a) Direct visualization of GltPh transport domain elevator movements by HS-AFM. Left: A typical HS-AFM image of a GltPh reconstituted membrane displays densely packed GltPh trimers (dashed outline). Right: Conformational dynamics of a representative GltPh trimer under substrate-free conditions (imaging rate: 1 s−1, frame size: 20nm). Each GltPh protomer in the trimer (top) shows reversible conformational alternation between outward facing (up, U) and inward facing (down, D) states (bottom). (b) HS-AFM movie frames of D96N bacteriorhodopsin (bR) exposed to repeated dark and green light illumination cycles (imaging rate: 1 s−1). bR trimers are highlighted by the white dashed triangles. Under illumination, conformational changes result in significant changes in the topography, notably a movement of the E-F loop outwards from the 3-fold axis (E-F loops of neighboring activated trimers interact closely in the activated state).
