Figure 2.
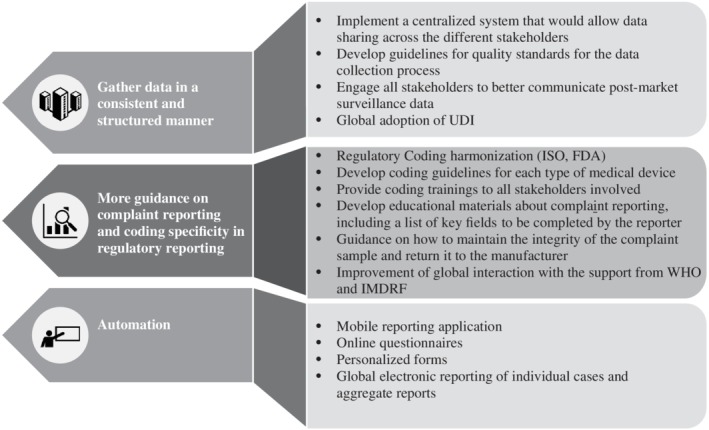
Recommendations to obtain better postmarket complaint data for medical devices. FDA, Food and Drug Administration; IMDRF, International Medical Device Regulators Forum; ISO, International Organization for Standardization; UDI, Unique Device Identifier; WHO, World Health Organization
