Scheme 14.
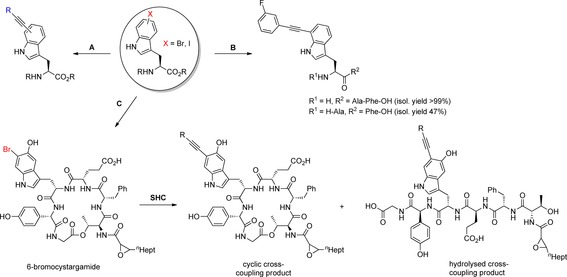
Late‐stage diversification by SHC with halotryptophans as well as halotryptophan containing peptides and natural products. A) Diversification of unprotected bromo‐ or iodotryptophans. Reaction conditions: alkyne (3.0 equiv), PdCl2(CH3CN)2 (5 mol %), sXPhos (15 mol %), Cs2CO3 (2.5 equiv), water/acetonitrile 1:1, 100 °C, μwave, 2 h. R=phenyl, 3‐fluorophenyl, 4‐cyanophenyl, 3‐thiopheneyl, 2‐hydroxyethyl, 2‐phenylethyl, 4‐ethynylbutyl, cyclohexylmethyl. B) Diversification of 7‐bromotryptophan containing tripeptides with 3‐fluorophenylacetylene. Reaction conditions: See A). C) Production of 6‐bromocystargamide by precursor‐directed biosynthesis with K. cystarginea. Subsequent SHC was performed with semi‐pure starting material; partial hydrolysis of cyclic cross‐coupling product to the linear analogue was observed. R=3‐fluorophenyl.67
