Scheme 15.
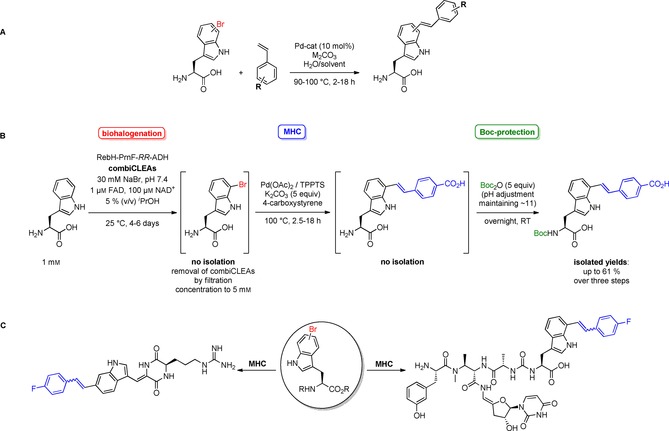
MHC with unprotected bromotryptophans and bromotryptophan containing natural products. A) i) Pd(OAc)2 (10 mol %), TPPTS (30 mol %), K2CO3 (1.5 equiv), styrene (5 equiv), water/(dioxane), 100 °C, 18 h; R=H, 4‐carboxy, 4‐methoxy, 4‐trifluoromethyl, 3‐fluoro, 3‐nitro;68 ii) Na2PdCl4 (10 mol %), TXPTS (23 mol %), Na2CO3 (4 equiv), styrene (2 equiv), water/(acetonitrile), 90 °C, μwave, 2 h; R=H, 4‐methyl, 4‐amino, 4‐fluoro, 3‐fluoro, 4‐pyridyl; additionally, acrylic acid was used as coupling partner.69 B) Chemoenzymatic three‐step one‐pot approach combining enzymatic bromination, MHC and Nα‐Boc protection; Pd loading 10–20 mol %;68 C) MHC diversification of the natural products barettin (left) and 7‐bromopacidamycin (right); reaction conditions: see A) ii).69
