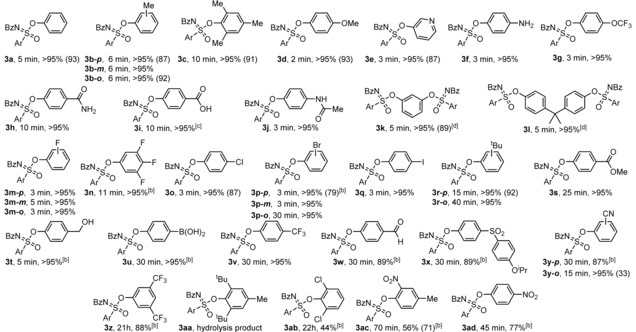
Table 1.
Scope of Si‐free SuFEx reactions of sulfonimidoyl fluoride 1 with phenolic derivatives.[a]
|
|
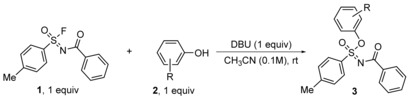
[a] Conversion was determined by 1H NMR measurements. Isolated yield in brackets. Reaction conditions for 1H NMR conversion: 1 (0.05 mmol), phenolic derivative 2 (1.05 equiv) and DBU (1.0 equiv) in CD3CN (0.55 mL), rt. For isolated yield: Fluoride 1 (0.5 mmol), phenolic derivatives 2 (0.5 mmol), DBU (1.5 mmol) in anhydrous CH3CN (1 mL), rt. [b] Hydrolysis by‐product was formed (see SI). [c] 2 equiv of DBU were used. [d] 2 equiv of 1 were used.