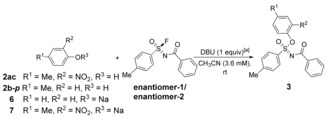
Table 3.
Enantioselective silicon‐free SuFEx reactions.[a]
|
Enantiomer[b] |
Phenol |
ee [c] |
|---|---|---|
|
enantiomer‐1 |
2 b‐p |
73 % (95[d]) |
|
2 ac |
<2 % |
|
|
6 |
>99 % |
|
|
|
7 |
>99 % |
|
|
|
|
|
enantiomer‐2 |
2 b‐p |
71 % (95[d]) |
|
2 ac |
<2 % |
|
|
6 |
>99 % |
|
|
7 |
>99 % |
[a] Reaction conditions: enantiomer‐1/enantiomer‐2 (1 mL, 3.6 mm in CH3CN), phenolic derivative (1 equiv), DBU (1 equiv), rt. When phenolate 6 or 7 was adopted as the substrate, DBU was not added. [b] enantiomer‐1 and enantiomer‐2 refer to two enantiomers with retention times of 16.8 and 18.8 min with chiral HPLC, respectively. [c] ee determined using chiral HPLC and calculated by ee= , [d] 10 equiv 2 b‐p used.
