Figure 4.
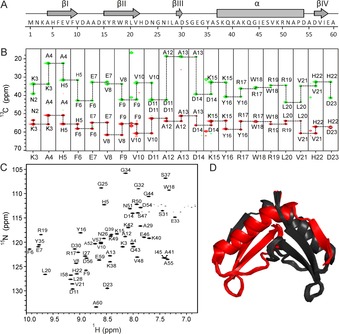
Structural characterization of SP‐22 small protein from H. volcanii. (PDB ID: 6Q2Z; BMRB ID: 34334). A) The amino acid sequence and the schematic representation of the secondary‐structure elements based on the solution‐state NMR spectroscopy structure. B) Sequential assignment for residues K3 to D23. The 3D HNCACB NMR spectrum was recorded at 700 MHz, 298 K; it contains 5 mm protein, 50 mm sodium phosphate buffer pH 7.5, 100 mm NaCl, 5 % D2O, 0.5 mm DSS. CA are shown in red and CB are highlighted in green. C) 1H,15N Best TROSY spectrum (600 MHz) of 5 mm small protein in 50 mm sodium phosphate buffer, pH 7.5, 100 mm NaCl, 95 % H2O/5 % D2O at 298 K. Backbone resonance assignment is indicated. D) Solution‐state NMR spectroscopy structure of SP‐22 protein. Ribbon representation of the best 20 structures is shown as a symmetrical dimer. The monomer consists of one α helix and four β‐sheet regions. Black and red represent two monomeric subunits. The figure was generated with PyMOL.50
