Abstract
Objective
This study aimed to evaluate ertugliflozin in patients with overweight and obesity with type 2 diabetes mellitus.
Methods
Data from three placebo‐controlled, randomized, Phase 3 studies were pooled. Patients with baseline BMI ≥ 25 (1,377/1,544; 89%) were assessed with a stratification by BMI subgroup.
Results
At week 26, reductions from baseline in glycated hemoglobin A1c (HbA1c), fasting plasma glucose, body weight (BW), and systolic blood pressure (SBP) were greater with ertugliflozin versus placebo. For placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg, respectively, least squares mean change was 0.1%, −0.8%, and −0.9% for HbA1c and −1.2 kg, −3.1 kg, and −3.2 kg for BW. HbA1c reductions were consistent across BMI subgroups. For ertugliflozin 5 mg and 15 mg, least squares mean change (placebo adjusted) in absolute BW was −1.4 kg and −1.2 kg for BMI 25 to < 30, −1.8 kg and −1.9 kg for BMI 30 to < 35, and −2.5 kg and −2.9 kg for BMI ≥ 35. Percent BW changes were similar across BMI subgroups. Incidence of adverse events was 52.5%, 44.6%, and 50.1% with placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg, respectively.
Conclusions
Meaningful reductions in HbA1c, fasting plasma glucose, BW, and SBP were observed with ertugliflozin in patients with overweight and obesity with type 2 diabetes mellitus. Ertugliflozin improved HbA1c and SBP and reduced BW across BMI subgroups. Ertugliflozin was generally well tolerated.
Study Importance.
What is already known?
-
►
Ertugliflozin, a selective sodium‐glucose cotransporter 2 inhibitor, is approved as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus (T2DM).
-
►
Ertugliflozin, alone or in combination with metformin or metformin and sitagliptin, significantly reduces glycated hemoglobin (HbA1c), fasting plasma glucose, body weight, and systolic blood pressure (SBP) in adults with T2DM.
What does this study add?
-
►
Clinically meaningful reductions in HbA1c, fasting plasma glucose, body weight, and SBP were observed with both ertugliflozin 5 mg and 15 mg in patients with overweight and obesity with T2DM. Reductions in HbA1c and SBP with ertugliflozin were consistent across BMI subgroups. Reductions in absolute body weight were observed across all BMI subgroups, including the subgroup of patients with the highest baseline BMI (≥ 35); percent change in body weight was similar across BMI subgroups.
-
►
Reductions in HbA1c were consistent across BMI subgroups, indicating that the glycemic efficacy of ertugliflozin is independent of baseline BMI.
-
►
Ertugliflozin was generally well tolerated in patients with overweight and obesity with T2DM.
Introduction
Diabetes is a major global health burden, affecting approximately 422 million adults, with 1.6 million deaths in 2016 directly caused by diabetes and another 2.2 million deaths attributable to high blood glucose in 2012 1. Approximately 90% of patients with type 2 diabetes mellitus (T2DM) are reported to have overweight or obesity 2. Weight loss of 5% to 10% is associated with significant improvement in glycemic control, lipids, and blood pressure (BP) in patients with T2DM with overweight or obesity 3. In addition, a randomized controlled study evaluating the effects of weight loss targets of 5%, ~10%, and ~15% and weight maintenance in patients with obesity (BMI 37.9 [SD 4.3]) demonstrated that even a moderate weight loss of 5% improved metabolic function in organs such as the adipose tissue, liver, and muscle, with progressively greater weight reduction leading to dose‐dependent changes in the main adipose tissue biological pathways 4. Therapeutic options that not only improve glycemic and metabolic outcomes for patients with T2DM and obesity but also reduce body weight are therefore desirable 5. Diet and lifestyle interventions designed to achieve and maintain 5% weight loss are advised for all patients with T2DM with overweight or obesity 6. When selecting pharmacologic treatments for patients with overweight or obesity with T2DM, the American Diabetes Association 5, 6 and European Association for the Study of Diabetes 5 recommend antihyperglycemic agents (AHAs) that promote weight loss or that are weight neutral. Metformin added to lifestyle measures is the preferred initial glucose‐lowering medication in newly diagnosed patients with T2DM. The choice of subsequent AHA is important, as some of the available therapies (for example, thiazolidinediones, sulfonylureas, and glinides) often result in weight gain 7 and others such as dipeptidyl peptidase 4 inhibitors are weight neutral 8, whereas glucagon‐like peptide 1 (GLP‐1) receptor agonists 9 and sodium‐glucose cotransporter 2 (SGLT2) inhibitors 10, 11 have demonstrated weight reduction. SGLT2 inhibitors act through an insulin‐independent mechanism to reduce renal tubular glucose reabsorption, preventing excessive blood glucose from returning to the circulatory system, with subsequent elimination through the urine 12. As a consequence, glycemia is reduced in patients with T2DM. Weight loss associated with SGLT2 inhibition appears to be a result of the renal excretion of glucose and the resulting caloric loss in the urine 11. Several studies have assessed the glycemic efficacy of different AHAs in patients with overweight or obesity with differing results 13, 14, 15, 16, 17, 18. However, a meta‐analysis conducted by Cai et al. 19 suggested that the baseline BMI was not associated with glycemic efficacy of different AHA regimens (including metformin, sulfonylureas, alpha‐glucosidase inhibitors, thiazolidinediones, dipeptidyl peptidase 4 inhibitors, GLP‐1 agonists, and SGLT2 inhibitors) in groups of patients with T2DM categorized by baseline BMI.
Ertugliflozin, a selective SGLT2 inhibitor 20, 21, is approved as an adjunct to diet and exercise to improve glycemic control in adults with T2DM 22, 23. Clinical studies in the eValuation of E RTugliflozin effIcacy and Safety (VERTIS) Phase 3 program have demonstrated that ertugliflozin alone or in combination with metformin or metformin and sitagliptin not only significantly reduces glycated hemoglobin A1c (HbA1c) and fasting plasma glucose (FPG) but is also associated with reductions in body weight and BP in adults with T2DM 24, 25, 26, 27, 28, 29, 30. However, the association between baseline BMI and the efficacy of ertugliflozin has not been evaluated comprehensively. The aim of this current analysis was to evaluate pooled data from three placebo‐controlled Phase 3 studies in the VERTIS Phase 3 program 24, 29, 30 to assess the efficacy and safety of ertugliflozin in patients with overweight and obesity with T2DM. A subgroup analysis of glycemic efficacy, changes in body weight, and systolic BP (SBP) in patients categorized by baseline BMI (25 to < 30, 30 to < 35, and ≥ 35) was included.
Methods
Study design and treatment
Data for this post hoc analysis were pooled from three randomized, double‐blind, multicenter, placebo‐controlled, Phase 3 studies (VERTIS SITA2, VERTIS MET, VERTIS MONO) 24, 29, 30 of a total of 1,544 patients with T2DM treated with ertugliflozin or placebo. Studies had similar overall study populations, designs, and enrollment criteria (Supporting Information Table S1). This analysis focused on the subgroup of patients with overweight and obesity (baseline BMI ≥ 25) and included an analysis stratified according to baseline BMI (25 to < 30, 30 to < 35, and ≥ 35). All studies contributing data to this analysis were conducted in accordance with principles of Good Clinical Practice and were approved by the appropriate institutional review boards and regulatory agencies. Informed consent was obtained from individuals in each study.
Details of inclusion criteria, exclusion criteria, and study design for the individual studies have been reported previously 27, 29, 30. Briefly, adults with T2DM were randomized (1:1:1) to treatment with placebo, ertugliflozin 5 mg, or ertugliflozin 15 mg once daily for 26 weeks. Inclusion criteria included BMI ≥ 18.0 for all three placebo‐controlled Phase 3 studies; the VERTIS MET study also had an upper limit for BMI of 40.0. Exclusion criteria included unstable body weight (defined as ≥ 5% change in body weight in the previous 6 months), receipt of a weight loss medication or other medication associated with weight changes, or bariatric surgery (within 12 months of screening, or more than 12 months prior to screening and was not weight stable for VERTIS MONO and VERTIS SITA2, or at any time in the past for VERTIS MET).
Patients received glycemic rescue therapy if they exceeded protocol‐defined FPG thresholds. Patients in each study received counseling on diet, and they were asked to maintain a routine exercise program with consistent physical activity throughout the study. Body weight was measured with a standardized digital scale. Measurements were taken in duplicate at approximately the same time of day after voiding, and the mean of the two readings was used in the analysis. Sitting BP was measured in triplicate with an automated oscillometric BP measuring device. Laboratory assessments were performed at a central laboratory.
End points and assessments
Efficacy
The efficacy end points reported for this post hoc pooled analysis were the changes from baseline in HbA1c, FPG, body weight, and SBP at week 26. The proportions of patients meeting specific targets for each efficacy end point (HbA1c < 7.0%, body weight reduction from baseline of ≥ 5%, and SBP < 130 mm Hg [among patients with baseline SBP ≥ 130 mm Hg]) at week 26 were reported. The changes from baseline in triglycerides, total cholesterol, low‐density lipoprotein cholesterol (LDL‐C), and high‐density lipoprotein cholesterol (HDL‐C) at week 26 were also assessed.
Safety
Safety and tolerability of ertugliflozin across the pooled studies were assessed through the overall incidence of adverse events (AEs) and serious AEs, the incidence of prespecified AEs of interest for SGLT2 inhibitors (symptomatic hypoglycemia and AEs of urinary tract infection, genital mycotic infection [GMI; by gender], and hypovolemia), and the incidence of AEs related to osmotic diuresis.
Statistical analysis
Analyses of data from patients with baseline BMI ≥ 25 from the three placebo‐controlled studies were performed post hoc.
Efficacy analysis
Efficacy analyses excluded results obtained after initiation of glycemic rescue therapy. The least squares (LS) means for change from baseline in HbA1c, FPG, body weight, SBP, and lipids (triglycerides, total cholesterol, LDL‐C, and HDL‐C) with ertugliflozin at week 26 were calculated and compared with placebo using a longitudinal data analysis model with fixed effects for study, treatment, time, baseline estimated glomerular filtration rate (eGFR, continuous), and treatment‐by‐time interaction with baseline constrained to be the same across treatments. Time was treated as a categorical variable. The proportion of patients with HbA1c < 7%, body weight reduction ≥ 5%, and SBP < 130 mm Hg at week 26 was measured using the Miettinen and Nurminen method 31, with missing data at week 26 considered as “nonresponders.” LS mean changes from baseline in HbA1c, body weight (absolute and percent), and SBP were also analyzed by baseline BMI subgroup as follows: 25 to < 30, 30 to < 35, and ≥ 35. For these end points for each BMI subgroup, a repeated‐measures ANCOVA model was used. The repeated‐measures ANCOVA model was adjusted for treatment, time, study, baseline eGFR, baseline value of the response variable, and the interaction of time by treatment. Time was treated as a categorical variable. All subgroup analyses were considered exploratory rather than inferential. CIs are based on the nominal 95% level, and no correction for multiple testing was made.
Safety analysis
The safety analyses were conducted in the population of all randomized and treated patients with baseline BMI ≥ 25 from across the three studies. The number and percentage of randomized treated patients with AEs were determined, including data obtained after initiation of glycemic rescue therapy, except for the analysis of hypoglycemia, which excluded results obtained after initiation of glycemic rescue. GMIs by gender, urinary tract infections, symptomatic hypoglycemia, and hypovolemia were prespecified AEs of special interest.
Results
Patients
Overall, 1,377 (89% of the total pooled population) patients with baseline BMI ≥ 25 were included in this analysis; 37.0% (509/1,377) had baseline BMI of 25 to < 30, 35.7% (492/1,377) had baseline BMI of 30 to < 35, and 27.3% (376/1,377) had baseline BMI of ≥ 35. Baseline characteristics for patients with BMI ≥ 25 were similar across treatment groups (Table 1). The mean (SD) age was 57.0 (9.5) years, and baseline eGFR was 89.0 (18.4) mL/min/1.73 m2. The duration of T2DM was 7.2 (5.7) years, and HbA1c was 8.1% (0.9%). The baseline body weight was 91.5 (19.1) kg, and BMI was 32.5 (5.3).
Table 1.
Baseline demographics and disease characteristics
| Placebo | Ertugliflozin 5 mg | Ertugliflozin 15 mg | Total | |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 241 (53.8) | 243 (52.2) | 241 (52.1) | 725 (52.7) |
| Female | 207 (46.2) | 223 (47.9) | 222 (48.0) | 652 (47.4) |
| Total | 448 (100.0) | 466 (100.0) | 463 (100.0) | 1,377 (100.0) |
| Age, y, mean (SD) | 56.6 (9.5) | 57.0 (9.6) | 57.4 (9.5) | 57.0 (9.5) |
| Duration of T2DM, y, mean (SD) | 7.0 (5.6) | 7.1 (5.8) | 7.4 (5.6) | 7.2 (5.7) |
| Race, n (%) | ||||
| White | 349 (77.9) | 365 (78.3) | 346 (74.7) | 1,060 (77.0) |
| Asian | 48 (10.7) | 47 (10.1) | 62 (13.4) | 157 (11.4) |
| Black or African American | 28 (6.3) | 31 (6.7) | 36 (7.8) | 95 (6.9) |
| American Indian or Alaskan | 6 (1.3) | 1 (0.2) | 4 (0.9) | 11 (0.8) |
| Multiple | 17 (3.8) | 22 (4.7) | 15 (3.2) | 54 (3.9) |
| Weight (kg), mean (SD) a | 92.2 (20.4) | 92.1 (19.7) | 90.4 (17.1) | 91.5 (19.1) |
| BMI (kg/m2), mean (SD) | 32.5 (5.5) | 32.6 (5.5) | 32.3 (5.0) | 32.5 (5.3) |
| 25 to < 30, n (%) | 165 (36.8) | 170 (36.5) | 174 (37.6) | 509 (37.0) |
| 30 to < 35, n (%) | 157 (35.0) | 171 (36.7) | 164 (35.4) | 492 (35.7) |
| ≥ 35, n (%) | 126 (28.1) | 125 (26.8) | 125 (27.0) | 376 (27.3) |
| HbA1c (%), mean (SD) b | 8.1 (0.9) | 8.1 (0.9) | 8.2 (1.0) | 8.1 (0.9) |
| FPG (mg/dL), mean (SD) c | 174.0 (41.6) | 172.5 (45.7) | 172.0 (44.3) | 172.8 (43.9) |
| SBP (mm Hg), mean (SD) d | 130.5 (14.4) | 131.1 (13.2) | 131.1 (12.7) | 130.9 (13.4) |
| eGFR (mL/min/1.73 m2), mean (SD) e | 89.4 (19.0) | 88.6 (17.6) | 89.1 (18.7) | 89.0 (18.4) |
n = 448 for placebo, 463 for ertugliflozin 5 mg, and 463 for ertugliflozin 15 mg.
n = 447 for placebo, 462 for ertugliflozin 5 mg, and 457 for ertugliflozin 15 mg.
n = 439 for placebo, 454 for ertugliflozin 5 mg, and 455 for ertugliflozin 15 mg.
n = 440 for placebo, 460 for ertugliflozin 5 mg, and 456 for ertugliflozin 15 mg.
eGFR estimated using Modification of Diet in Renal Disease equation.
eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus.
Efficacy
Glycemic control
At week 26, there was a reduction in HbA1c from baseline with ertugliflozin 5 mg and 15 mg compared with an increase with placebo. The LS mean change (95% CI) from baseline in HbA1c was 0.1% (95% CI: 0.0% to 0.1%) for placebo, −0.8% (−0.8% to −0.7%) for ertugliflozin 5 mg, and −0.9% (−1.0% to −0.8%) for ertugliflozin 15 mg (Figure 1A). In the subgroup analysis by baseline BMI, the LS mean change from baseline in HbA1c was similar across all BMI subgroups (Figure 2A).
Figure 1.
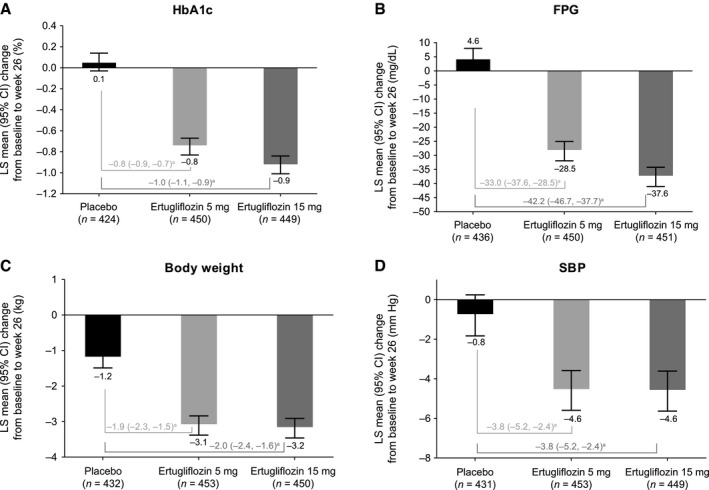
LS mean change from baseline at week 26 in (A) HbA1c, (B) FPG, (C) body weight, and (D) SBP. aPlacebo‐adjusted difference in LS means (95% CI).
Figure 2.
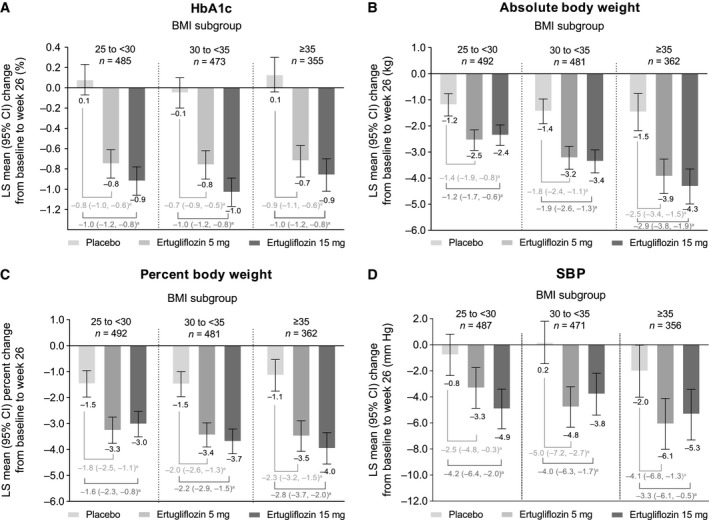
LS mean change from baseline at week 26 in (A) HbA1c, (B) absolute body weight, (C) percent body weight, and (D) SBP by BMI subgroup. aPlacebo‐adjusted difference in LS means (95% CI). Mean (SD) baseline HbA1c was 8.1% (0.9%), 8.0% (0.9%), and 8.2% (1.1%) for placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg for patients with baseline BMI 25 to <30; 8.1% (0.9%), 8.1% (0.9%), and 8.2% (1.0%) for placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg for patients with baseline BMI 30 to <35; and 8.0% (0.8%), 8.1% (0.9%), and 8.1% (0.8%) for placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg for patients with baseline BMI ≥35.Mean (SD) baseline body weight was 77.5 (10.7) kg, 77.6 (11.5) kg, and 76.2 (9.4) kg for placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg for patients with baseline BMI 25 to <30; 91.1 (12.4) kg, 90.9 (12.1) kg, and 91.4 (11.2) kg for placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg for patients with baseline BMI 30 to <35; and 109.6 (21.2) kg, 111.3 (20.2) kg, and 106.5 (15.9) kg for placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg for patients with baseline BMI ≥35.Mean (SD) baseline SBP was 128.6 (15.5) mm Hg, 130.1 (13.9) mm Hg, and 129.7 (12.9) mm Hg for placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg for patients with baseline BMI 25 to <30; 131.9 (12.6) mm Hg, 131.2 (12.8) mm Hg, and 131.3 (13.0) mm Hg for placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg for patients with baseline BMI 30 to <35; and 131.4 (14.8) mm Hg, 132.4 (12.5) mm Hg, and 132.6 (11.8) mm Hg for placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg for patients with baseline BMI ≥35.
At week 26, a higher proportion of patients with BMI ≥ 25 who received ertugliflozin 5 mg or 15 mg had HbA1c < 7.0% compared with placebo (32.3%, 40.7%, and 15.9%, respectively; Figure 3A). The difference in the proportion of patients with HbA1c < 7.0% at week 26 relative to placebo was 16.4 percentage points (95% CI: 10.9 to 21.8) for ertugliflozin 5 mg and 24.8 percentage points (95% CI: 19.1 to 30.4) for ertugliflozin 15 mg.
Figure 3.

Proportion of patients at week 26 with (A) HbA1c < 7%, (B) body weight reduction ≥ 5%, or (C) SBP < 130 mm Hg (among patients with a baseline SBP ≥ 130 mm Hg).
At week 26, there was a reduction in FPG from baseline with ertugliflozin 5 mg and 15 mg compared with an increase with placebo (Figure 1B).
Body weight
At week 26, the reduction in body weight from baseline was greater with ertugliflozin 5 mg and 15 mg compared with placebo. The LS mean change (95% CI) from baseline was −1.2 kg (−1.5 to −0.9) for placebo, −3.1 kg (−3.4 to −2.8) for ertugliflozin 5 mg, and −3.2 kg (−3.5 to −2.9) for ertugliflozin 15 mg (Figure 1C). In the subgroup analysis according to baseline BMI, the LS mean change (95% CI) from baseline in absolute body weight was −1.2 kg (−1.6 to −0.8) for placebo, −2.5 kg (−2.9 to −2.2) for ertugliflozin 5 mg, and −2.4 kg (−2.8 to −2.0) for ertugliflozin 15 mg in the BMI 25 to < 30 subgroup; −1.4 kg (−1.9 to −1.0) for placebo, −3.2 kg (−3.7 to −2.8) for ertugliflozin 5 mg, and −3.4 kg (−3.8 to −2.9) for ertugliflozin 15 mg in the BMI 30 to < 35 subgroup; and −1.5 kg (−2.2 to −0.8) for placebo, −3.9 kg (−4.6 to −3.3) for ertugliflozin 5 mg, and −4.3 kg (−5.0 to −3.7) for ertugliflozin 15 mg in the BMI ≥ 35 subgroup (Figure 2B). The LS mean percent change (95% CI) from baseline in body weight was similar across all BMI subgroups as follows: −1.5% (−2.0% to −1.0%) for placebo, −3.3% (−3.8% to −2.8%) for ertugliflozin 5 mg, and −3.0% (−3.5% to −2.5%) for ertugliflozin 15 mg in the BMI 25 to < 30 subgroup; −1.5% (−2.0% to −1.0%) for placebo, −3.4% (−3.9% to −3.0%) for ertugliflozin 5 mg, and −3.7% (−4.2% to −3.2%) for ertugliflozin 15 mg in the BMI 30 to < 35 subgroup; and −1.1% (−1.8% to −0.5%) for placebo, −3.5% (−4.1% to −2.9%) for ertugliflozin 5 mg, and −4.0% (−4.6% to −3.4%) for ertugliflozin 15 mg in the BMI ≥ 35 subgroup (Figure 2C).
At week 26, a higher proportion of patients with BMI ≥ 25 who received ertugliflozin 5 mg or 15 mg had body weight reductions of ≥ 5% from baseline compared with placebo (28.1%, 28.5%, and 10.7%, respectively; Figure 3B). The difference in the proportion of patients with body weight reductions of ≥ 5% (95% CI) from baseline to week 26 relative to placebo was 17.4 percentage points (12.4 to 22.4) for ertugliflozin 5 mg and 17.8 percentage points (12.8 to 22.8) for ertugliflozin 15 mg.
BP
At week 26, the reduction in SBP from baseline was greater with ertugliflozin 5 mg and 15 mg compared with placebo. The LS mean change (95% CI) from baseline was −0.8 mm Hg (−1.8 to 0.2) for placebo, −4.6 mm Hg (−5.6 to −3.6) for ertugliflozin 5 mg, and −4.6 mm Hg (−5.6 to −3.6) for ertugliflozin 15 mg (Figure 1D). In the subgroup analysis according to baseline BMI, the reduction in SBP was similar across all BMI subgroups as follows: −0.8 mm Hg (−2.4 to 0.8) for placebo, −3.3 mm Hg (−4.9 to −1.8) for ertugliflozin 5 mg, and −4.9 mm Hg (−6.5 to −3.4) for ertugliflozin 15 mg in the BMI 25 to < 30 subgroup; 0.2 mm Hg (−1.5 to 1.8) for placebo, −4.8 mm Hg (−6.3 to −3.2) for ertugliflozin 5 mg, and −3.8 mm Hg (−5.4 to −2.2) for ertugliflozin 15 mg in the BMI 30 to < 35 subgroup; and −2.0 mm Hg (−4.0 to 0.0) for placebo, −6.1 mm Hg (−8.0 to −4.1) for ertugliflozin 5 mg, and −5.3 mm Hg (−7.3 to −3.3) for ertugliflozin 15 mg in the BMI ≥ 35 subgroup (Figure 2D).
At week 26, a higher proportion of patients with BMI ≥ 25 who received ertugliflozin 5 mg or 15 mg had SBP < 130 mm Hg (among those with SBP ≥ 130 mm Hg at baseline) compared with placebo (36.3%, 37.0%, and 19.1%, respectively; Figure 3C). The difference in the proportion of patients with BMI ≥ 25 and with SBP ≥ 130 mm Hg at baseline who subsequently had SBP < 130 mm Hg at week 26 (95% CI) relative to placebo was 17.1 percentage points (9.2 to 24.9) for ertugliflozin 5 mg and 17.9 percentage points (9.9 to 25.8) for ertugliflozin 15 mg.
Changes in lipids
Changes from baseline to week 26 in triglycerides, total cholesterol, LDL‐C, and HDL‐C are shown in Figure 4.
Figure 4.

LS mean change from baseline at week 26 in (A) triglycerides, (B) total cholesterol, (C) LDL‐C, and (D) HDL‐C. a Placebo‐adjusted difference in LS means (95% CI). Mean (SD) baseline triglyceride level was 182.3 (117.0) mg/dL, 177.2 (109.5) mg/dL, and 169.0 (106.2) mg/dL for placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg.Mean (SD) baseline total cholesterol level was 180.4 (41.8) mg/dL, 179.6 (41.7) mg/dL, and 176.8 (41.3) mg/dL for placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg.Mean (SD) baseline LDL‐C level was 98.3 (34.7) mg/dL, 97.8 (34.4) mg/dL, and 96.7 (34.2) mg/dL for placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg.Mean (SD) baseline HDL‐C level was 46.0 (11.9) mg/dL, 46.7 (13.1) mg/dL, and 46.7 (11.4) mg/dL for placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg.
Safety
In patients with BMI ≥ 25, the incidence of overall AEs, serious AEs, and discontinuations because of AEs was similar with ertugliflozin 5 mg, 15 mg, and placebo (Table 2). No patients died during the initial 26 weeks of the studies included in this pooled analysis. There was a higher incidence of GMIs with ertugliflozin 5 mg and 15 mg, particularly among females, compared with placebo (Table 2).
Table 2.
Summary of adverse events
| Patients | Placebo | Ertugliflozin 5 mg | Ertugliflozin 15 mg |
|---|---|---|---|
| n (%) | 448 (32.5) | 466 (33.8) | 463 (33.6) |
| With ≥ 1 AE | 235 (52.5) | 208 (44.6) | 232 (50.1) |
| With ≥ 1 SAE | 13 (2.9) | 13 (2.8) | 9 (1.9) |
| Discontinued treatment because of an AE | 7 (1.6) | 9 (1.9) | 7 (1.5) |
| Who died | 0 | 0 | 0 |
| With AEs of special interest | |||
| GMI (female) a | 7 (3.4) | 22 (9.9) | 28 (12.6) |
| GMI (male) b | 1 (0.4) | 9 (3.7) | 9 (3.7) |
| UTI | 19 (4.2) | 20 (4.3) | 20 (4.3) |
| Symptomatic hypoglycemia | 9 (2.0) | 13 (2.8) | 15 (3.2) |
| Hypovolemia | 8 (1.8) | 3 (0.6) | 4 (0.9) |
n = 207 for placebo, 223 for ertugliflozin 5 mg, and 222 for ertugliflozin 15 mg.
n = 241 for placebo, 243 for ertugliflozin 5 mg, and 241 for ertugliflozin 15 mg.
AE, adverse event; GMI, genital mycotic infection; SAE, serious adverse event; UTI, urinary tract infection.
Discussion
The current analysis evaluated the impact of ertugliflozin in patients with overweight and obesity with T2DM, using pooled data from patients with a baseline BMI ≥ 25 enrolled in three placebo‐controlled clinical studies. Clinically meaningful reductions in HbA1c, FPG, body weight, and SBP were observed with both ertugliflozin 5 mg and 15 mg. The efficacy of ertugliflozin on reductions in HbA1c, body weight, and SBP was demonstrated across all baseline BMI subgroups assessed (25 to < 30, 30 to < 35, and ≥ 35). Although no formal comparison was made, the reductions from baseline at week 26 in HbA1c, SBP, and body weight observed in patients with BMI ≥ 25 were similar to those observed in the overall pooled population 32.
Reductions in HbA1c were consistent across BMI subgroups, indicating that the glycemic efficacy of ertugliflozin is independent of baseline BMI. This finding is aligned with the results of a meta‐analysis comparing the effects of different AHAs, including SGLT2 inhibitors (dapagliflozin, empagliflozin, ipragliflozin, and canagliflozin), in groups of patients categorized according to baseline BMI 19. In this meta‐analysis, treatment with SGLT2 inhibitors (vs. placebo) was associated with a significant decrease in HbA1c both in patients with a baseline BMI ≥ 25 to < 30 (weighted mean difference: −0.64%; 95% CI: −0.64 to −0.63%; P < 0.001) and in patients with a baseline BMI ≥ 30 (weighted mean difference: −0.60%; 95% CI: −0.70 to −0.51%; P < 0.001). Regression analysis indicated that the effect of the AHAs evaluated, including SGLT2 inhibitors, on HbA1c was independent of baseline BMI.
In this current analysis, there was a decrease in absolute body weight from baseline with ertugliflozin across BMI subgroups ranging from 2.4 to 4.3 kg. While greater absolute weight loss was associated with higher BMI, this appeared to be a reflection of higher baseline body weight, as the percentage change in body weight was similar across BMI subgroups (~3‐4% with ertugliflozin in each BMI subgroup). These findings indicate that patients with overweight and obesity, including those in the highest BMI category (≥ 35), can benefit from weight loss with ertugliflozin. In a recent meta‐analysis, body weight significantly decreased in patients with T2DM who received different dosages of SGLT2 inhibitors (dapagliflozin, empagliflozin, canagliflozin, ipragliflozin, tofogliflozin, and luseogliflozin) compared with patients who received a placebo, with a greater reduction in body weight with higher doses of SGLT2 inhibitors 33. Despite the observed maintenance of weight loss in patients with T2DM treated with SGLT2 inhibitors, the observed reduction in body weight tends to plateau after approximately 26 weeks despite sustained urinary glucose excretion, with observed weight loss less than predicted based on caloric loss from urinary glucose excretion 34, 35. Factors including compensatory increases in calorie intake and/or changes in energy expenditure may contribute to this attenuated weight loss. Combining SGLT2 inhibitors with other drugs that have different mechanisms of action to improve weight loss might be more effective. For example, a combination of dapagliflozin with the GLP‐1 receptor agonist exenatide, which suppresses appetite, resulted in a greater mean reduction in body weight in patients with T2DM than with either agent alone 36. Another study in individuals with overweight or obesity but without T2DM demonstrated meaningful reductions in body weight with canagliflozin coadministered with phentermine, a sympathomimetic amine that also suppresses appetite 34. Further studies are warranted to evaluate potential use of SGLT2 inhibitors in combination with other agents for long‐term weight management in patients with T2DM.
High BP and abnormal lipid levels are common comorbidities in patients with T2DM with poor glycemic control 37. In this analysis, clinically meaningful reductions in SBP were observed with both ertugliflozin 5 mg and 15 mg with over one‐third of patients with a baseline SBP above 130 mm Hg having an SBP below 130 mm Hg at week 26. One meta‐analysis has suggested that a reduction in SBP to below 135 mm Hg was associated with a 10% reduction in all‐cause mortality and a 17% reduction in the risk of stroke 38. In this analysis, ertugliflozin was associated with a reduction in triglycerides and a small increase in LDL‐C, HDL‐C, and total cholesterol. Other studies with SGLT2 inhibitors have also reported small increases in LDL‐C 39, 40.
Ertugliflozin was generally well tolerated in patients with T2DM and a baseline BMI ≥ 25. The incidence of urinary tract infection, symptomatic hypoglycemia, and hypovolemia‐related AEs was low and similar across treatment groups. Of the prespecified AEs of interest for SGLT2 inhibitors, only the incidence of genital mycotic infections in both men and women was higher with ertugliflozin than placebo. This is consistent with the safety data for the total population in the placebo‐pooled population 32 and is to be expected, as an increased incidence of GMIs is a known class effect of SGLT2 inhibitors because of the associated glucosuria. These findings are consistent with previously published efficacy and safety analyses of the overall pooled data from the three placebo‐controlled studies of ertugliflozin 32.
There are several limitations with this analysis, including its post hoc nature and the short‐term nature of the studies. Longer‐term studies of ertugliflozin have shown that weight loss is maintained for up to 104 weeks 41, 42. While ertugliflozin reduced body weight in patients with overweight and obesity over the 26‐week study period, it is unclear as to how this weight reduction might affect adipocyte and adipose tissue function and translate into long‐term benefits. Longer‐term outcomes with ertugliflozin will be assessed in the ongoing VERTIS cardiovascular outcomes trial (NCT01986881) 43. There was also a lack of within‐BMI subgroup randomization of treatment, although patient numbers and baseline characteristics were similar between treatment groups for patients with BMI ≥ 25. Small patient numbers in the BMI subgroups could reduce the reliability of the statistical analyses.
Conclusion
In patients with overweight and obesity with T2DM, treatment with ertugliflozin for 26 weeks led to greater reductions from baseline in HbA1c, FPG, body weight, and SBP compared with placebo. More patients receiving ertugliflozin achieved the metabolic goals of reduction in HbA1c to < 7%, weight loss ≥ 5%, or SBP < 130 mm Hg compared with placebo. Improvement of HbA1c, reduction in body weight, and improvement of SBP were observed across BMI subgroups. The population of patients with overweight and obesity, including those in the highest BMI category (≥ 35), derived the same glycemic benefit as well as similar weight loss and SBP benefits from treatment with ertugliflozin. Ertugliflozin was generally well tolerated in patients with overweight and obesity with T2DM.
Funding agencies
This study was sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD), and Pfizer Inc., New York, NY, USA. Medical writing support was provided by Marion James of Engage Scientific Solutions and was funded by MSD in collaboration with Pfizer Inc.
Disclosure
SBH has received fees for serving on advisory boards from Tanita and Medifast. SG and SGT are employees of Pfizer Inc. and may own shares/stock options in Pfizer Inc. JL, AP, and AR are employees of MSD and may own stock in Merck & Co., Inc., Kenilworth, NJ, USA. HH was an employee of MSD at the time of the analysis and may own stock in Merck & Co., Inc., Kenilworth, NJ, USA.
Clinical trial registration
ClinicalTrials.gov identifiers NCT01958671, NCT02033889, and NCT02036515.
Supporting information
Acknowledgments
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the United States and/or European Union or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
[Copyright corrected on 16 April 2020 after initial online publication.]
References
- 1. World Health Organization . Global Report on Diabetes. Geneva: WHO; 2016. [Google Scholar]
- 2. Bramante CT, Lee CJ, Gudzune KA. Treatment of obesity in patients with diabetes. Diabetes Spectr 2017;30:237‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chukir T, Shukla AP, Saunders KH, Aronne LJ. Pharmacotherapy for obesity in individuals with type 2 diabetes. Expert Opin Pharmacother 2018;19:223‐231. [DOI] [PubMed] [Google Scholar]
- 4. Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab 2016;23:591‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Diabetes Association . 8. Obesity management for the treatment of type 2 diabetes: Standards of Medical Care in Diabetes‐2019 . Diabetes Care 2019;42(suppl 1):S81‐S89. [DOI] [PubMed] [Google Scholar]
- 7. Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA 2010;303:1410‐1418. [DOI] [PubMed] [Google Scholar]
- 8. Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase‐4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta‐analysis. BMJ 2012;344:e1369. doi: 10.1136/bmj.e1369 [DOI] [PubMed] [Google Scholar]
- 9. Hinnen D. Glucagon‐like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr 2017;30:202‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ribola FA, Cançado FB, Schoueri JH, De Toni VF, Medeiros VH, Feder D. Effects of SGLT2 inhibitors on weight loss in patients with type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci 2017;21:199‐211. [PubMed] [Google Scholar]
- 11. Lee PC, Ganguly S, Goh SY. Weight loss associated with sodium‐glucose cotransporter‐2 inhibition: a review of evidence and underlying mechanisms. Obes Rev 2018;19:1630‐1641. [DOI] [PubMed] [Google Scholar]
- 12. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016;134:752‐772. [DOI] [PubMed] [Google Scholar]
- 13. Diels J, Angermund R, Schroeder M, Worbes‐Cerezo M, Thompson G. The efficacy and effectiveness in HBA1C‐lowering is dependent on baseline body mass index (BMI) for sitagliptin but not canagliflozin in the treatment of type 2 diabetes mellitus (T2DM). Value Health 2014;17:A334. doi: 10.1016/j.jval.2014.08.639 [DOI] [PubMed] [Google Scholar]
- 14. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427‐2443. [DOI] [PubMed] [Google Scholar]
- 15. Gavin LA, Barth J, Arnold D, Shaw R. Troglitazone add‐on therapy to a combination of sulfonylureas plus metformin achieved and sustained effective diabetes control. Endocr Pract 2000;6:305‐310. [DOI] [PubMed] [Google Scholar]
- 16. Monami M, Cremasco F, Lamanna C, Marchionni N, Mannucci E. Predictors of response to dipeptidyl peptidase‐4 inhibitors: evidence from randomized clinical trials. Diabetes Metab Res Rev 2011;27:362‐372. [DOI] [PubMed] [Google Scholar]
- 17. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560‐2572. [DOI] [PubMed] [Google Scholar]
- 18. Zoungas S, Chalmers J, Kengne AP, et al. The efficacy of lowering glycated haemoglobin with a gliclazide modified release‐based intensive glucose lowering regimen in the ADVANCE trial. Diabetes Res Clin Pract 2010;89:126‐133. [DOI] [PubMed] [Google Scholar]
- 19. Cai X, Yang W, Gao X, Zhou L, Han X, Ji L. Baseline body mass index and the efficacy of hypoglycemic treatment in type 2 diabetes: a meta‐analysis. PLoS One 2016;11:e0166625. doi: 10.1371/journal.pone.0166625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kalgutkar AS, Tugnait M, Zhu T, et al. Preclinical species and human disposition of PF‐04971729, a selective inhibitor of the sodium‐dependent glucose cotransporter 2 and clinical candidate for the treatment of type 2 diabetes mellitus. Drug Metab Dispos 2011;39:1609‐1619. [DOI] [PubMed] [Google Scholar]
- 21. Miao Z, Nucci G, Amin N, et al. Pharmacokinetics, metabolism, and excretion of the antidiabetic agent ertugliflozin (PF‐04971729) in healthy male subjects. Drug Metab Dispos 2013;41:445‐456. [DOI] [PubMed] [Google Scholar]
- 22. European Medicines Agency . Steglatro™ (Ertugliflozin) Summary of Product Characteristics. Hoddesdon, UK: Merck Sharp & Dohme Ltd; 2018. [Google Scholar]
- 23. US Food and Drug Administration . SteglatroTM (Ertugliflozin) Prescribing Information. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2017. [Google Scholar]
- 24. Dagogo‐Jack S, Liu J, Eldor R, et al. Efficacy and safety of the addition of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sitagliptin: The VERTIS SITA2 placebo‐controlled randomized study. Diabetes Obes Metab 2018;20:530‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grunberger G, Camp S, Johnson J, et al. Ertugliflozin in patients with stage 3 chronic kidney disease and type 2 diabetes mellitus: the VERTIS RENAL randomized study. Diabetes Ther 2018;9:49‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hollander P, Liu J, Hill J, et al. Ertugliflozin compared with glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin: The VERTIS SU randomized study. Diabetes Ther 2018;9:193‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller S, Krumins T, Zhou H, et al. Ertugliflozin and sitagliptin co‐initiation in patients with type 2 diabetes: The VERTIS SITA randomized study. Diabetes Ther 2018;9:253‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pratley RE, Eldor R, Raji A, et al. Ertugliflozin plus sitagliptin versus either individual agent over 52 weeks in patients with type 2 diabetes mellitus inadequately controlled with metformin: The VERTIS FACTORIAL randomized trial. Diabetes Obes Metab 2018;20:1111‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenstock J, Frias J, Páll D, et al. Effect of ertugliflozin on glucose control, body weight, blood pressure and bone density in type 2 diabetes mellitus inadequately controlled on metformin monotherapy (VERTIS MET). Diabetes Obes Metab 2018;20:520‐529. [DOI] [PubMed] [Google Scholar]
- 30. Terra SG, Focht K, Davies M, et al. Phase III, efficacy and safety study of ertugliflozin monotherapy in people with type 2 diabetes mellitus inadequately controlled with diet and exercise alone. Diabetes Obes Metab 2017;19:721‐728. [DOI] [PubMed] [Google Scholar]
- 31. Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med 1985;4:213‐226. [DOI] [PubMed] [Google Scholar]
- 32. Liu J, Tarasenko L, Terra SG, et al. Efficacy of ertugliflozin in monotherapy or combination therapy in patients with type 2 diabetes: a pooled analysis of placebo‐controlled studies. Diab Vasc Dis Res 2019;16:415‐423. [DOI] [PubMed] [Google Scholar]
- 33. Cai X, Yang W, Gao X, et al. The association between the dosage of SGLT2 inhibitor and weight reduction in type 2 diabetes patients: a meta‐analysis. Obesity (Silver Spring) 2018;26:70‐80. [DOI] [PubMed] [Google Scholar]
- 34. Hollander P, Bays HE, Rosenstock J, et al. Coadministration of canagliflozin and phentermine for weight management in overweight and obese individuals without diabetes: a randomized clinical trial. Diabetes Care 2017;40:632‐639. [DOI] [PubMed] [Google Scholar]
- 35. Pereira MJ, Eriksson JW. Emerging role of SGLT‐2 inhibitors for the treatment of obesity. Drugs 2019;79:219‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frias JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION‐8): a 28 week, multicentre, double‐blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016;4:1004‐1016. [DOI] [PubMed] [Google Scholar]
- 37. Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004;291:335‐342. [DOI] [PubMed] [Google Scholar]
- 38. Bangalore S, Kumar S, Lobach I, Messerli FH. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and bayesian random‐effects meta‐analyses of randomized trials. Circulation 2011;123:2799‐2810. [DOI] [PubMed] [Google Scholar]
- 39. Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy and safety of sodium‐glucose co‐transporter‐2 inhibitors in type 2 diabetes mellitus: systematic review and network meta‐analysis. Diabetes Obes Metab 2016;18:783‐794. [DOI] [PubMed] [Google Scholar]
- 40. Xiong W, Xiao MY, Zhang M, Chang F. Efficacy and safety of canagliflozin in patients with type 2 diabetes: a meta‐analysis of randomized controlled trials. Medicine (Baltimore) 2016;95:e5473. doi: 10.1097/MD.0000000000005473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hollander P, Hill J, Johnson J, et al. Results of VERTIS SU extension study: safety and efficacy of ertugliflozin treatment over 104 weeks compared to glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin. Curr Med Res Opin 2019;35:1335‐1343. [DOI] [PubMed] [Google Scholar]
- 42. Gallo S, Charbonnel B, Goldman A, et al. Long‐term efficacy and safety of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: 104‐week VERTIS MET trial. Diabetes Obes Metab 2019;21:1027‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cannon CP, McGuire DK, Pratley R, et al. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS‐CV). Am Heart J 2018;206:11‐23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


