Figure 3.
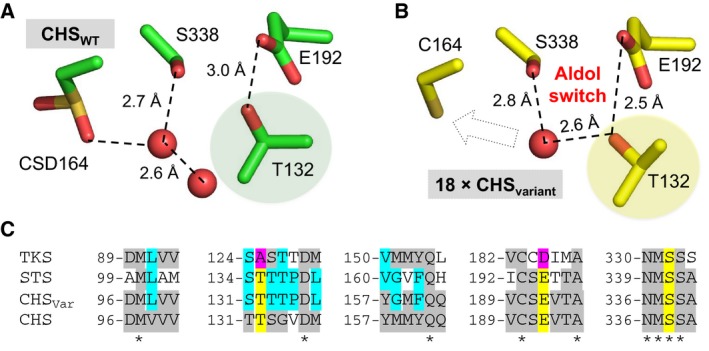
Overview of the aldol switch in type III polyketide synthases. Hydrogen‐bonding network of the active site water proposed to be the catalytic base in the cleavage of the thioester bond between Cys164 and the tetraketide intermediate in (A) wild‐type CHS (PDB: http://www.rcsb.org/pdb/search/structidSearch.do?structureId=1BI5) and (B) variant CHS with STS‐like activity (18 residues altered; PDB: http://www.rcsb.org/pdb/search/structidSearch.do?structureId=1U0W) 12. CSD is a modified cysteine visible in the crystal structure. The dashed arrow in the bottom panel indicates the potential interaction between the catalytic water and Cys164. The shaded residues show the significant differences in orientation of Thr132, the basis of the ‘aldol switch’. (C) Sequence alignment of four type III polyketide synthases within the active site region. Residues highlighted in yellow and magenta are involved in the STS‐like aldol switch, the latter showing differences seen in TKS. CHSVar = variant CHS with STS‐like activity 12.
