Figure 1.
Multipotent Myelofibrosis Hematopoietic Stem and Progenitor Cells (HSPCs) Are Biased for Megakaryocyte Differentiation
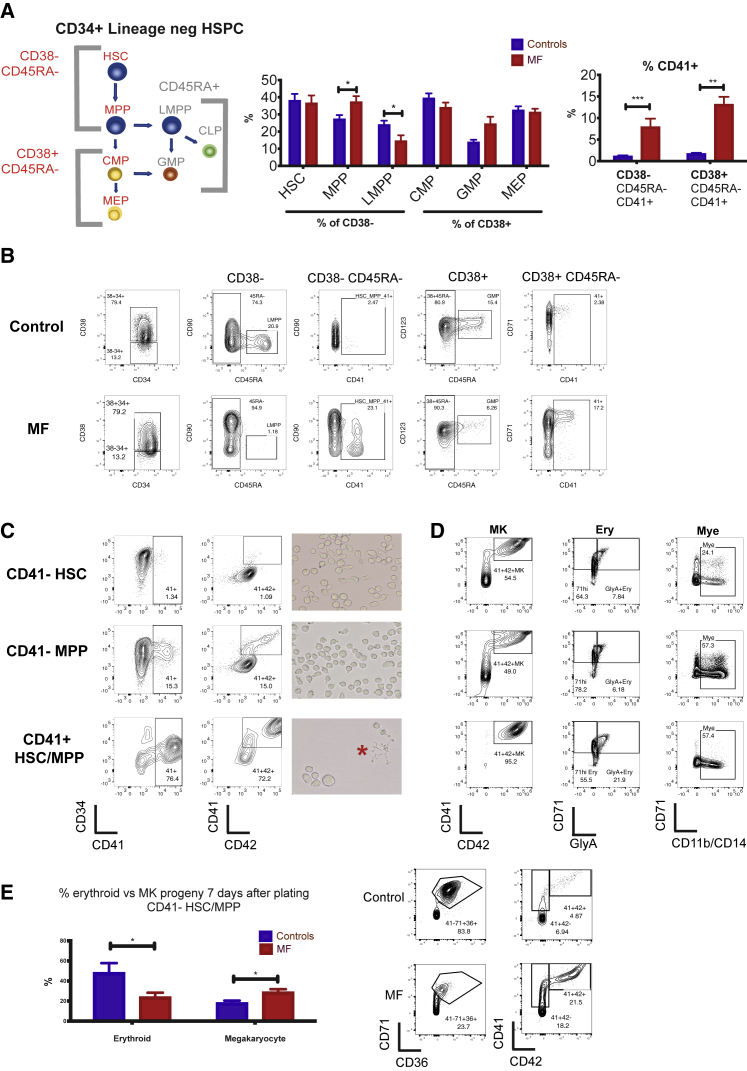
(A) Left: model of classically defined CD34+ lin− HSPC subpopulations, in which multi-potent cells (HSCs, hematopoietic stem cells; MPPs, multi-potent progenitor cells; LMPPs, lymphoid-primed multi-potent progenitors) are CD38− and down-stream progenitors (CMPs, common myeloid progenitors; MEPs, megakaryocyte-erythroid progenitors; GMPs, granulocyte-monocyte progenitors) are CD38+. CD45RA+ populations (LMPP/GMP) do not have erythroid or megakaryocyte potential. Middle: % of each classically defined HSPC population in the CD34+ lin− compartment, demonstrating increased MPPs and reduced LMPPs in myelofibrosis (MF) compared to controls. Right: % cells expressing CD41, a surface antigen previously shown to identify cells with increased potential for megakaryocyte differentiation, is increased in both CD38− CD45RA− (HSC/MPP) and CD38+ CD45RA− (CMP/MEP) compartments in myelofibrosis (MF patients, N = 23; controls, N = 14, see also Table S1).
(B) Representative FACS plot of a healthy donor control and myelofibrosis patient showing gating strategies.
(C) Left: FACS analysis of CD41− HSC (top), CD41− MPP (middle), and CD41+ HSC/MPP (bottom) from healthy donors cultured in megakaryocyte differentiation media (with added recombinant human TPO and stem cell factor [SCF]). CD41+ HSC/MPP demonstrate increased potential for megakaryocyte differentiation, with faster acquisition of the mature megakaryocyte antigen CD42 at an early time point (day 6). Right: images of cultures showing enlarged cell size and proplatelet formation (red star) indicative of accelerated megakaryocyte differentiation from CD41+ HSC/MPP. Representative examples of 3 replicate experiments shown.
(D) FACS analysis of CD41− HSC, CD41− MPP and CD41+ HSC/MPP from healthy donors cultured for 12–14 days in megakaryocyte (MK), erythroid (Ery), or myeloid (Mye) differentiation media. CD41+ HSC/MPP showed a higher % of mature CD41+42+ megakaryocytes and glycophorin A+ CD71+ erythroblasts and equivalent CD11b/CD14+ myeloid cells versus CD41− fractions. Representative examples of 3 replicate experiments shown. % of total live (7AAD-), single cells shown.
(E) Summary chart (left) and representative FACS plots (right) showing percentage of myelofibrosis and control CD41− HSC/MPP cultured in “bi-potent” erythroid and megakaryocyte differentiation media that give rise to megakaryocyte versus erythroid progeny 6 days after plating (gated on live cells). (controls, n = 7; myelofibrosis [MF], n = 8). Charts show mean + SEM,∗∗∗p < 0.001; ∗∗p ≤ 0.01; ∗p < 0.05). See also Figure S1.