Abstract
Insulin has been a life-saving drug for millions of people with diabetes. However, several challenges exist which limit therapeutic benefits and reduce patient convenience. One key challenge is the fibrillation propensity, which necessitates refrigeration for storage. To address this limitation, we chemically synthesized and evaluated a methylene thioacetal human insulin analog (SCS-Ins). The synthesized SCS-Ins showed enhanced serum stability and aggregation resistance while retaining bioactivity compared with native insulin.
Graphical Abstract
INTRODUCTION
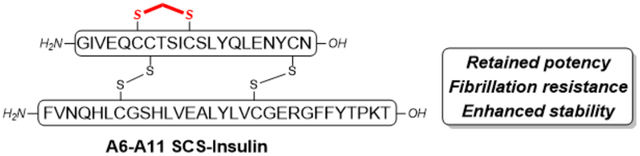
Insulin is a 51-amino acid peptide connected by two separate chains and three disulfide bonds (one intra-chain, A6-A11 and two inter-chain, A7-B7 & A20-B19).1 Ever since its discovery in 1921, insulin has been the mainstay for the treatment of type 1 diabetes and late-stage type 2 diabetes.2 Rapid- and long-acting insulin analogs have been developed over the past decades to achieve better blood glucose control.3 However, these analogs still suffer from the undesired properties of native insulin such as propensity of degradation, fibrillation, and disulfide scrambling, which makes them difficult for storage and transport, particularly in circumstances where refrigeration is challenging.4 Therefore, the development of novel insulin analogs with enhanced stability and without the need of cold-chain delivery is highly desired.5
Due to this need, a number of strategies were reported recently to increase insulin stability. First, an additional disulfide bond between A10-B4 was introduced to native insulin to generate a four-disulfide analog, which demonstrates reduced aggregation propensity while preserving insulin bioactivity (Figure 1A).6 Next, a single-chain insulin analog with a 6-residue linker connecting the C-terminal B chain and N-terminal A chain also led to reduced insulin fibrillation compared to native insulin (Figure 1B).7 Furthermore, using O-mannosylation at B27 threonine, the Tan group reported an insulin analog with reduced proteolysis and self-association (Figure 1C).8 The Tirrell group recently reported that mutation of B28 proline to a non-natural (4S)-hydroxyproline (Hzp) led to a stable analog with reduced aggregation through an additional hydrogen bond between Hzp and a backbone amide carbonyl group.9
Figure 1.
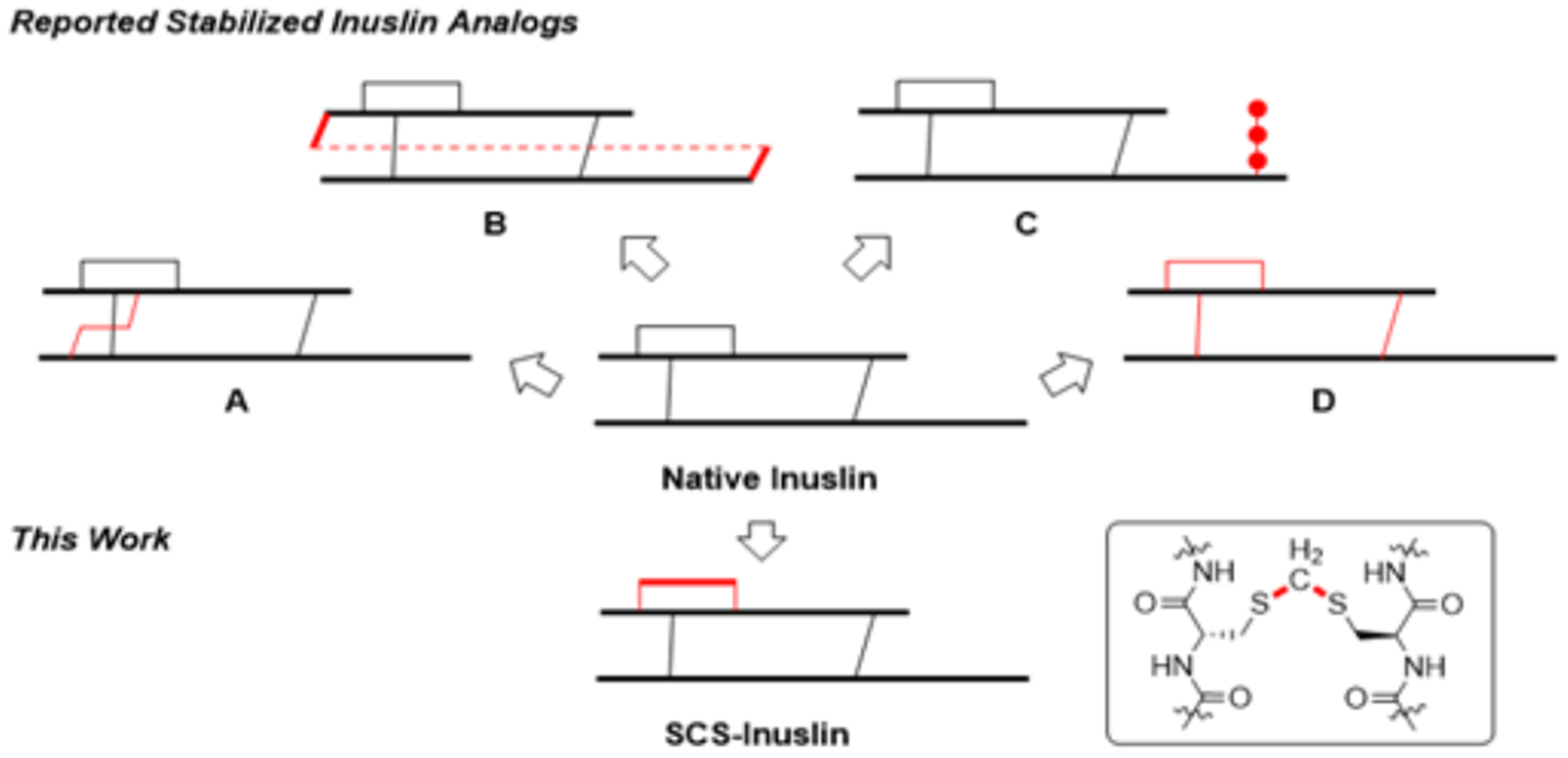
Top: Previously reported strategies to stabilize insulin. Bottom: The present work to introduce a methylene thioacetal to replace A6-A11 disulfide bond.
Recently, disulfide mimetics have been introduced to insulin with a goal to prevent disulfide reduction, scrambling and further side reactions, and thus increase stability. However, the introduced disulfide mimetics may influence insulin bioactivity depending on the extent of the structural perturbation.10 Among the reported studies, insulin analogs with A6-A11 thioether,11 A6-A11 cis-dicarba12 or A6-A1113 & A7-B714 diselenide bond replacement preserved insulin bioactivity. On the other hand, replacements involving A7-B7 triazole15 or A6-A11 trans-dicarba12 resulted in reduced potency (Figure 1D). While these analogs have some desired properties, non-natural amino acids are required, which makes practical applications challenging. We are especially interested in bridging the disulfide bond with carbon linkers to increase peptide stability.16 In this particular case, inserting a minimal linker into the disulfide bond is key to maintain the structural integrity and preserve the bioactivity of insulin. The Cramer group recently reported an elegant biocompatible solution phase synthesis of methylene thioacetal bond as a disulfide mimic and applied this method to the synthesis of SCS-oxytocin which shows enhanced stability and retained bioactivity.17 In this brief article we present our chemical synthesis and characterization of an A6-A11 methylene thioacetal bridge replaced human insulin analog (Figure 1).
RESULTS AND DISCUSSION
The chemical synthesis of insulin and insulin analogs is still a daunting task even at the current stage largely due to the low yield in competing disulfide pairing and the notorious hydrophobicity of the A chain.18 Several methods have been developed for improving the A chain solubility to facilitate synthesis.19 We carried out our initial attempt by using an isoacyl peptide approach developed by Liu et al. (Scheme 1).20 The partially protected A chain S1 [isoacyl A8-A9, A7-tBu, A20-Acm] was synthesized from 2-chlorotrityl chloride (2-CTC) resin. The free thiols in Cysteine A6 and A11 were intended to be converted into a methylene thioacetal. Following Cramer’s protocol, the peptide was dissolved and treated with Tris(2-carbox-yethyl)phosphine hydrochloride (TCEP.HCl) and potassium carbonate followed by trimethylamine (Et3N) and diiodomethane. A white precipitate S2 was generated; however, it is poorly soluble and chromatographically intractable (see supporting information for details). This result indicated that a simultaneous O to N acyl shift occurred during the reaction under basic condition, which removes the amine handle in the case of the isoacyl peptide, leading to reduced solubility.
Scheme 1.
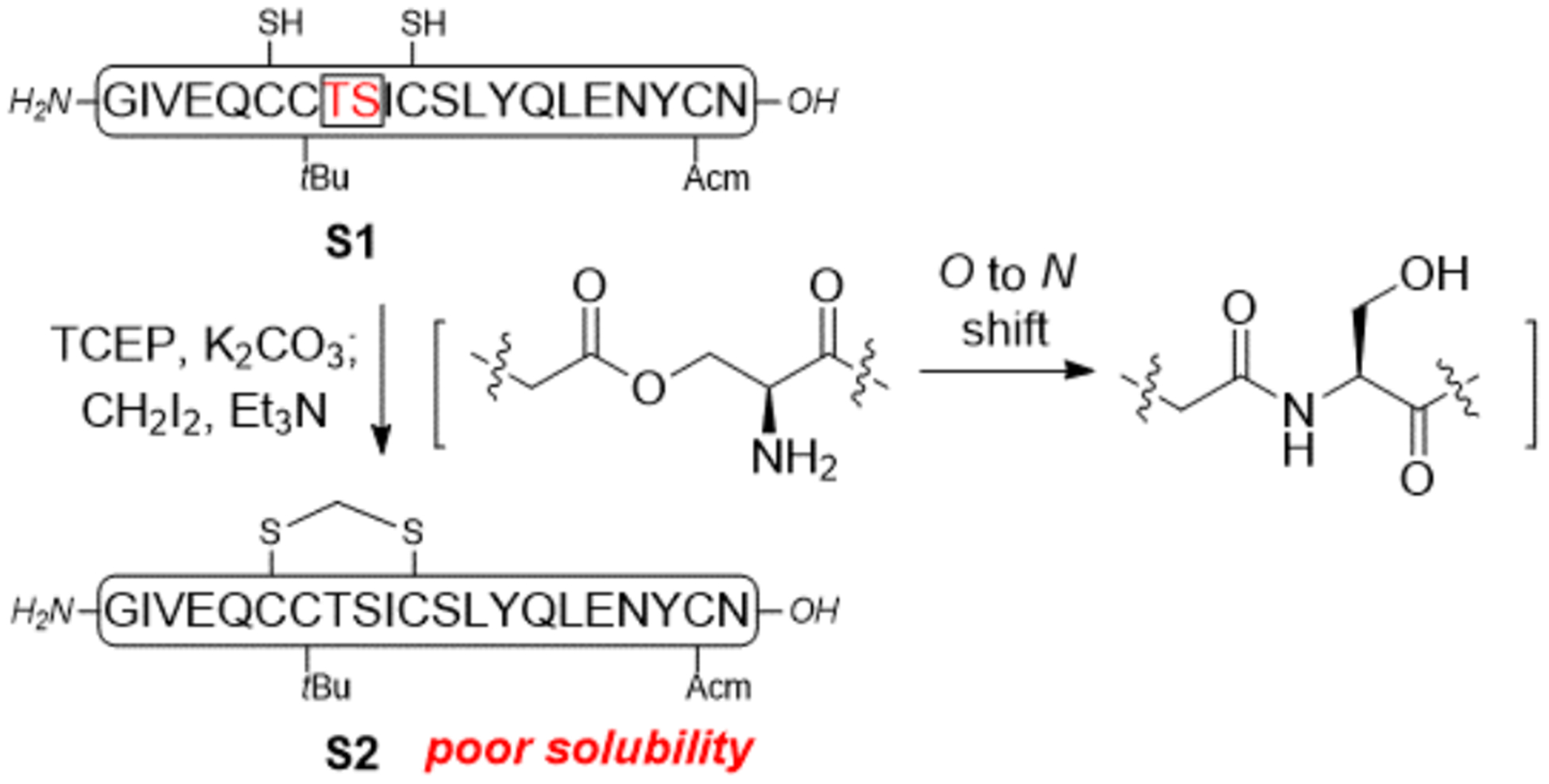
Initial attempt to synthesize the SCS-Insulin A chain using an isoacyl peptide strategy
To circumvent the labile nature of the isoacyl peptide under basic reaction conditions, we previously reported the use of Fmoc-Ddae-OH connecting N-terminal amine with a solubility tag through a hydrazine sensitive enamine bond.21 While Fmoc-Ddae-OH as a “helping hand” successfully solved the problem, its viscous oil nature presents a technical challenge during chemical synthesis. Our collaborator, Kay group further developed a 2nd generation helping hand, Fmoc-Ddap-OH (hh), as white powder with the replacement of a PEG linker to a hexyl linker.22 Our synthetic route using this helping hand approach towards the SCS-Ins is described in Scheme 2. First, the partially protected A chain [A7-Acm, A20-Mob] A1 was synthesized on 2-CTC resin through Fmoc SPPS. The N-terminal Gly- at A1 position was treated with Fmoc-Ddap-OH (hh) in methylpyrrolidone (NMP) to form an enamine linked complex A2. Additional SPPS to install hexa-lysine sequence followed by cleavage generates highly soluble A3, a key intermediate to circumvent the hydrophobicity of insulin A chain. We next reacted A3 with the Cramer’s methylene thioacetal formation condition. As expected, A3 showed greatly improved solubility in water and successfully yielded product A4, which also exhibited drastically increased solubility compared with S2 (see supporting information for detail). We did not detect any hydrolysis product during the reaction, which further highlights the strength of this helping hand method. A4 was then treated with a cocktail of TFA/TIPS/H2O at 45 °C to remove the A20-Mob and yielded the corresponding A20-thiol product A5 in 60% yield for two steps from purified A3.23 Next, protected B chain [B7-Acm, B19-Trt] B1 was generated through Fmoc-SPPS and activated with DTDP during acidic cleavage to give the [B7- Acm, B19-SPy] chain B2.20
Scheme 2.
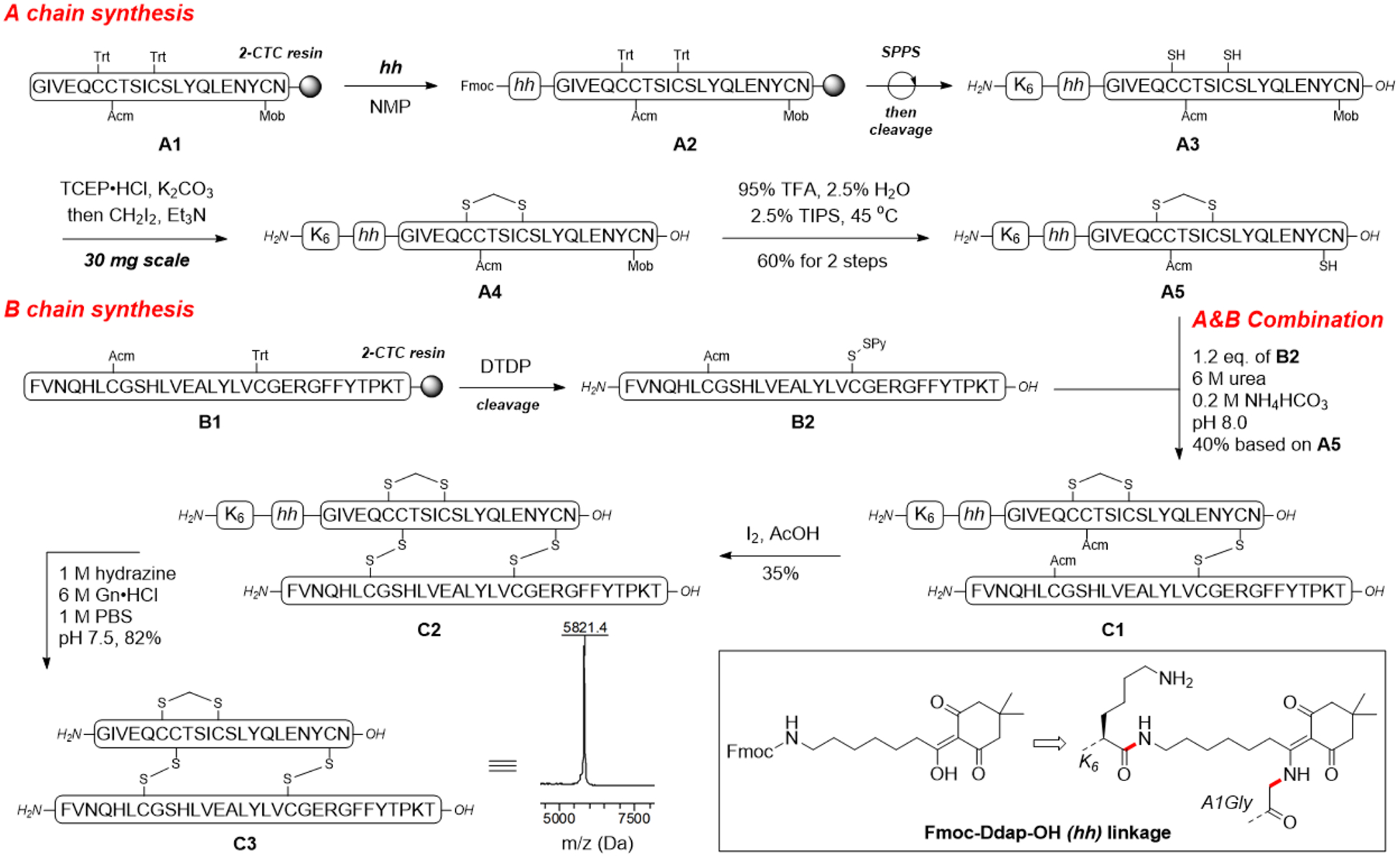
Chemical synthesis of SCS-Insulin enabled by N-terminal helping hand strategy
With both SCS-A and B chains in hand, we then investigated the formation of the two inter-chain disulfide bonds. To this end, A5 and B2 were dissolved and stirred in a 6 M urea containing 0.2 M NH4HCO3 (pH 8.0) buffer solution. The reaction reached complete conversion of A5 in 20 min and yielded a heterodimer C1 in 40% yield based on A5 after HPLC purification. Conversion of C1 to C2 (hh-tagged SCS-Ins) was smoothly achieved by treatment with the excess amount of iodine in AcOH and the A7-B7 disulfide bond was formed through an in situ Acm deprotection-disulfide formation manner. Again, we did not detect any de-tagged products during the two-step combination. To complete the synthesis, C2 was treated with hydrazine to remove the helping hand tag. The product SCS-Ins C3 was obtained in 82% yield after purification (Scheme 2). The observed mass of C3 corresponded to the calculated mass with three crosslinks. The correct folding of two inter-chain disulfide bonds was further confirmed by proteinase Glu-C digestion and the fragments were characterized by LC-MS (Supporting information).24
The A6-A11 intra-chain disulfide bond plays a critical role in forming and stabilizing the receptor binding A chain N-terminal α-helix as a conformational constraint, which is important to facilitate the formation of two inter-chain disulfide bonds as well as the stability of the whole molecule.25 This rationale is supported by previous folding studies of porcine proinsulin and a recent study of reduced insulin A and B chain self-assembly experiment.26 Therefore, to evaluate whether this methylene thioacetal replacement influences insulin properties, we performed a series of experiments to compare native insulin with SCS-Ins side-by-side. First, to determine if thioacetal linkage has an effect on the secondary structure, circular dichroism (CD) spectra were obtained (Figure 2A). SCS-Ins has the α-helical secondary structure consistent with native insulin, indicating that the secondary structure is similar between the two molecules. We further used a phospho-AKT (Ser 473) insulin signaling stimulation assay to evaluate insulin bioactivity (Figure 2B). SCS-Ins has 2-fold reduced bioactivity (EC50 4.01 nM) than native insulin (EC50 1.91 nM) suggesting that the thioacetal linkage does not greatly influence insulin activity as SCS-Ins is still comparably potent. This in vitro result is further strengthened by an insulin tolerance test (ITT) in mice. Briefly, equal amount of native insulin and SCS-Ins (0.75 U/Kg) was subcutaneously injected in C57BL/6 mice and blood glucose levels were measured. As shown in Figure 2C, the two insulin molecules have similar glucose lowering effect, suggesting a comparable in vivo activity.
Fig. 2.
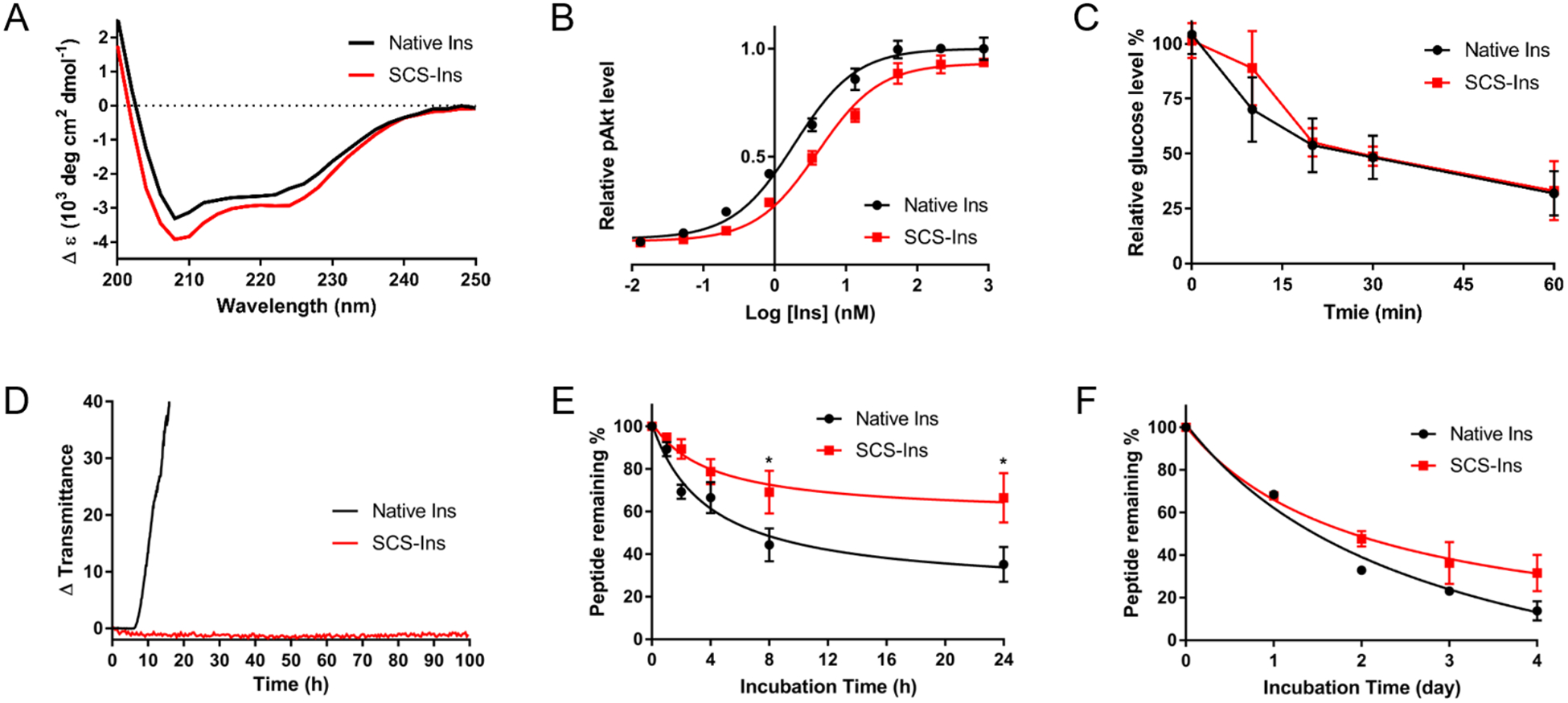
Characteristics of SCS-Ins in comparison with native Insulin. A) Circular dichroism spectra measured at 25 °C with a concentration of 0.2 mg/mL in pH 7.4 PBS. B) Human insulin signaling activation (Akt phosphorylation) assay. Data represent the average of 4 independent measurements and error bar represents SD. calc EC50 (95% CI): [Native Insulin = 1.91 (1.36–2.69) nM; SCS-Ins = 4.01 (2.91–5.53) nM]. C) Insulin tolerance test in mice. Data represent average of 4 male and female mice and error bar represents SD. D) Kinetic profiling of fibrillation, monitoring change in transmittance (i.e., increase in turbidity) at 1.0 mg/mL under continuous agitation at 37 °C in pH 7.4 PBS. E) Serum stability comparison at 37 °C at 0.1 mg/mL insulin concentration in human serum. Data represent 3 replicates at each time point and error bar represents SD. F) Thermal stability assay at 60 °C with 0.1 mg/mL insulin in pH 7.4 PBS. Data represent 3 replicates at each data point and error bar represents SD. 95% CI = 95% confidence intervals; SD = standard deviation; *p<0.05, Student t (unpaired) test.
We next performed various stability assays for both insulin molecules. We first evaluated their propensity for fibrillation using a stressed aging assay consisting of continuous agitation in pH 7.4 buffer at 37 °C.27 As shown in Figure 2D, native insulin behaves similarly to previous reports as it aggregated in under 10 hours of shaking while SCS-Ins did not aggregate even after 100 hours of shaking. This result demonstrates that SCS-Ins has a superior effect over native insulin in preventing aggregation, a key factor for insulin formulation. It was reported that unfolding of the N-terminal helix of the A chain exposes a hydrophobic surface, which may lead to accelerated insulin fibrillation.28 Fixing the A6-A11 disulfide bond using the unreducible methylene thioacetal in our case may stabilize the N-terminal helix of the A chain, leading to reduced insulin fibrillation. We argue that this may be a valuable strategy for future efforts to achieve stable insulin analogs. Next, both native insulin and SCS-Ins were exposed to human serum at 37 °C. SCS-Ins has enhanced serum stability compared to native insulin (Figure 2E). The SCS-Ins was digested to 70% whereas native insulin was digested to 40% after being incubated for 24 hours. Similarly, SCS-Ins also showed increased thermal stability compared with native insulin when both were incubated in a PBS (pH 7.4) at 60 °C for 4 days (Figure 2F). Taken together, the SCS-Ins preserves the biological activity of native insulin and demonstrates superior aggregation, serum and thermal stability over native insulin.
CONCLUSIONS
In conclusion, we have completed the chemical synthesis of the A6-A11 methylene thioacetal human insulin analog (SCS- Ins). A successful application of the second generation “helping hand” linking a hexa-lysine moiety at the N-terminal A chain enabled a solubility increase and the tolerance of harsh basic condition used to form the target methylene thioacetal. Along with our previous work on using this helping hand strategy to synthesize chicken insulin, we believe that this approach is a valuable and generally applicable tool for the chemical synthesis of peptides or proteins with high hydrophobicity. Furthermore, SCS-Ins not only retained bioactivity both in vitro and in vivo but also exhibited enhanced stability, indicating that methylene thioacetal could serve as an ideal disulfide surrogate for insulin modification. On the basis of current research, modifications of other clinically used insulin analogs and attempts to synthesize the inter-chain methylene thioacetal replaced insulin analogs are currently underway.
EXPERIMENTAL SECTION
Chemicals.
All commercially available chemicals were purchased and used directly without further purification.
Animals.
All animal experiments were performed in accordance with NIH guide for the care and use of laboratory animals and approved by the Institutional Animal Care and Use Committee (IACUC) of University of Utah.
Automated solid phase peptide synthesis.
Peptides were synthesized via Fmoc solid phase peptide synthesis on a commercial peptide synthesizer (Syzo I). The first amino acid was coupled manually to the 2-CTC resin (for 0.1 mmol peptide; 250 mg resin; 0.77 mmol/g) using 0.12 mmol of Fmoc-L-Asn(trt)-OH or Fmoc-L-Thr(tBu)-OH and 0.2 mmol of DIEA in 3.5 mL DCM and stirred at 25 °C for 1.5 h. The resin then was washed DMF and then DCM, followed by capping with a cocktail of DCM: MeOH: DIEA = 17:2:1 (3 min; 5 rounds) and finally washed DMF to get amino acid bonded resin (final loading = 0.4 mmol/g). Then the automated peptide synthesis was carried out in a 10 mL reactor vial with the following protocols for 0.1 mmol scale of both chains. Fmoc deprotection: 20% piperidine in DMF; Amino acid coupling: Fmoc protected amino acid (5.0 eq.), HATU (5.0 eq.), and DIEA (10.0 eq.) were used for the coupling steps.
Coupling of Fmoc-Ddap-OH linker.
For 0.1 mmol scale A chain bonded resin, 1.5 eq. of Fmoc-Ddap-OH dissolved in NMP (3 mL) was added and the total coupling required > 4 h agitation on rotator.22
Cleavage and Activation.
For Mob containing A chain resins, the cleavage was conducted using a cocktail consistent of 85% TFA, 5% TIS, 5% H2O and 5% thioanisole for 2 h at room temperature under gentle agitation on rotator. For B chain resins, the cleavage were conducted using a cocktail of 95% TFA, 2.5% TIS and 2.5% H2O containing 5.0 eq. of DTDP for 2.5 h at room temperature. The resin was then filtered off and the filtrate was precipitated by cold ethyl ether (40 mL) and cooled at −20 °C for 25 min. Following precipitation, the peptide was spun down on a centrifuge for 10 minutes at 3000 rcf. The supernatant was removed and the peptide pellet was subsequently washed 2x with 30 mL of cold ethyl ether. The peptide was dried under vacuum for 1 h before HPLC purification.
LC-MS analysis.
Characterization of peptides was performed by LC/MS on a Phenomenex Luna® 5 μm Phenyl-Hexyl (50 × 2 mm) 100 Å column at 0.4 mL/min with a water/acetonitrile gradient in 0.1% formic acid on an Agilent 6120 Quadrupole LC/MS system.
HPLC purification.
Preparative RP-HPLC of crude peptides was performed on Jupiter 5 μm C18 300 Å (250 × 10 mm) column at 3 mL/min with a water/acetonitrile gradient in 0.1% TFA from 5% aqueous ACN to 65% aqueous ACN over 45 minutes on an Agilent 1260 HPLC system.
Purity check.
Purity of SCS-Ins and native insulin samples for biological testing were determined to be >95% by analytical RP-HPLC performed on C18 column: Phenomenex, 150 mm × 4.6 mm, 100 Å, 5 μm at a flow rate 0.4 mL/min.
Synthesis of S2.
To a stirred solution of S1 (3.5 mg) in H2O (220 μL) and ACN (80 μL) was added a premixed solution of TCEP.HCl (780 μg, 2.0 eq.) and K2CO3 (760 μg, 4.0 eq.) in H2O (150 μL) in a dropwise manner. After stirring at room temperature for 1.5 h, Et3N (1.9 μL, 10.0 eq.) in THF (36 μL) was added to the mixture and followed with CH2I2 (1.0 μL, 6.0 eq.) in THF (36 μL). The mixture was then allowed to react shielded from light till a complete conversion of the starting material monitored by LCMS. The reaction was then quenched with 10% AcOH to a final pH of 6.5 then purified by HPLC. ESI M/Z (Da): Calc 1262.5 [M+2]+2; Found 1262.1 [M+2]+2.
Synthesis of A4.
The synthesis was conducted using standard condition in S2 synthesis with the replacement of A3 as substrate. ESI M/Z (Da): Calc 1202.6 [M+3]+3, 1803.5 [M+2]+2; Found 1202.7 [M+3]+3, 1803.7 [M+2]+2.
Synthesis of A5.
Purified A4 was dissolved and stirred at 45 °C in a cocktail (4 mL) consistent of 95% TFA, 2.5% TIS and 2.5% H2O. The reaction was monitored by LCMS till a complete consumption of A4 was reached (about 4 h). Then cold ethyl ether (40 mL) was added and the participated crude A5 was centrifuged and washed with cold ether (15 mL × 3). The collected crude A5 was dried in vacuum and purified by HPLC. Two-step yield was 60% based on A3. ESI M/Z (Da): Calc 1162.7 [M+3]+3, 1743.6 [M+2]+2; Found 1162.4 [M+3]+3, 1742.3 [M+2]+2.
Synthesis of C1.
Purified A5 (3.0 mg, 1.0 eq.) and B2 (3.7 mg, 1.2 eq.) was dissolved in a 0.2 M NH4HCO3 buffer containing 6 M urea (700 μL, pH 8.0) and vigorously stirred for 20 min at 25 °C. The reaction was detected by LCMS. When A5 was complete consumed, the reaction was diluted with 0.1% TFA ddH2O to 2 mL and the mixture was purified by HPLC. 40% yield based on A5. ESI M/Z (Da): Calc 1165.1 [M+6]+6, 1397.9 [M+5]+5, 1747.1 [M+4]+4; Found 1165.0 [M+6]+6, 1397.6 [M+5]+5, 1746.7 [M+4]+4.
Synthesis of C2.
To a stirred solution of C1 (7.0 mg, 1 eq.) in H2O containing 20% AcOH (900 μL) was added dropwise with freshly prepared I2 solution in AcOH (25 eq, 100 μL, 0.1 M) and the mixture was allowed to stir at 25 °C for 5 min. The oxidation was quenched with addition of 1 M ascorbic acid till decay of dark red color to colorless. Then the mixture was diluted with H2O and purified by HPLC. ESI M/Z (Da): Calc 978.4 [M+7]+7, 1141.3 [M+6]+6, 1369.4 [M+5]+5, 1711.5 [M+4]+4; Found 978.2 [M+7]+7, 1141.0 [M+6]+6, 1368.8 [M+5]+5, 1711.9 [M+4]+4.
Synthesis of C3 (SCS-Ins).
Purified C2 (4.0 mg) was dissolved and stirred in 500 μL of a 100 mM PBS (500 μL) consisting of 6 M Gn.HCl (pH 7.5) followed by addition of 100 mM PBS containing 2 M hydrazine pH 7.5 (500 μL). The linker and solubility tag were removed (about 30 min) and monitored by LCMS. Then the mixture was subsequently diluted with H2O and purified by HPLC. 82% yield; 6.9 % overall yield based on A3. Purity >95%. ESI M/Z (Da): Calc 971.3 [M+6]+6, 1165.3 [M+5]+5, 1456.4 [M+4]+4, 1941.6 [M+3]+3; Found 971.1 [M+6]+6, 1165.2 [M+5]+5, 1456.2 [M+4]+4, 1941.8 [M+3]+3. MALDI-MS (Da): Calc 5821.6; Found 5821.4.
Circular dichroism spectroscopy.
Circular dichroism spectroscopy was performed on an Applied Photophysics Chirascan Plus instrument. 0.2 mg/mL PBS (pH 7.4) solutions of both SCS-Ins and native insulin were prepared, and experiments were performed at 25 °C across a wavelength range from 200–250 nm. CD spectra were obtained with a step of 0.5 nm, 0.5 s per point, and the average of five scans with an averaged against five scan with PBS baseline subtracted.
Phospho-AKT (Ser 473) cell-based assay.
We followed a standard phosphorylation assay previously described.21b The pAkt Ser473 levels were measured in a mouse fibroblast cell line NIH 3T3, overexpressing human insulin receptor isoform B (IR-B). The cell line was cultured in DMEM containing 10% fetal bovine serum, 100 U/mL penicillin-streptomycin and 2.0 mg/mL puromycin. 100 μl (about 40,000 cells) per well were plated in a 96-well plates for each assay with culture media containing 1% FBS. 20 hours later, 50 μl of SCS-Ins or native insulin series dilution (0.003 to 860 nM) were pipetted into each well after the removal of the original media following 30-min incubation. Then the insulin solution was removed and the intracellular level of pAkt Ser473 was measured by HTRF pAkt Ser473 kit (Cisbio, Massachusetts, USA). The cells were first treated with cell lysis buffer (50 μl per well) for 1 h under mild shaking. 16 μL of cell lysate was then added to 4 μL of detecting reagent in a white 384-well plate. After 4h incubation, plate was read in a Synergy Neo plate reader and the data processed according to the manufacturer’s protocol. Each point is consisted of 4 replicates from two independent experiments.
In vitro human serum stability assay.
Each insulin sample was dissolved in a PBS (1,0 mg/mL, pH 7.4) and 100 μL of the dissolved solution was added into 900 μL of human serum from human male AB plasma (Sigma-Aldrich, sterile filtered, defrosted only once and pre-centrifuged 15 min at 13,000 rpm to remove lipid) at a final insulin concentration of 0.1 mg/mL. Tha mixture was then incubated at 37 °C water bath and individual 100 μL of the solution was taken up at certain, pre-determined time points (0, 1, 2, 4, 8, 24 h) and treated with 300 μL ACN containing 10% H2O and cooled on ice for 20 mins. The suspension was centrifuged at 13,000 rpm for 10 min at room temperature. Then 20 μL of supernatant was taken up and dissolved in 15 μL of 0.1% TFA containing H2O to make the HPLC sample. The sample was analyzed by RP-HPLC (injection volume = 30 μL; column: Phenomenex, 150 mm × 4.6 mm, 100 Å, μm) with a linear gradient of 5–50% B over 9 min (A: H2O + 0.1% TFA, B: ACN + 0.1% TFA; 0.4 mL/min flow rate). Peptide peak areas were integrated and the % of peptide left, compared to the initial was graphed against the time. The serum stability experiments were repeated 3 times for both SCS-Ins native insulin.
In vitro thermal stability assay.
Each insulin sample dissolved in PBS (0.1 mg/mL, pH 7.4). 200 μL of the dissolved solution was incubated at 60 °C (water bath) and individual 30 μL of the solution was taken up at day points (0, 1, 2, 3, 4 day) was analyzed by HPLC (column: Phenomenex, 150 mm × mm, 100 Å, 5 μm) with a linear gradient of 5–50% B over 8 min (0.4 mL/min flow rate). Peptide peak areas were integrated and the % of peptide left, compared to the initial was graphed against the time. The thermal stability experiments were repeated 3 times for both SCS-Ins and native insulin.
Insulin fibrillation assay.
We followed a standard method as previously described.27 Insulin samples were dissolved in pH 7.4 PBS to a final concentration of 1.0 mg/mL. Samples were plated at 150 μL per well (n=4/group) in a clear 96-well plate and sealed with optically clear and thermally stable seal. plate was immediately placed into an Infinite M200 plate reader and shaken continuously at 37 °C. Absorbance readings at 540 nm were collected every 6 min for 100 h, and absorbance values were subsequently converted to transmittance.
Insulin tolerance test.
ITT was performed on normal chow fed, 12–15 weeks’ old C57BL/6 mice with both genders. On the day of experiment, the mice were fasted for 4–6 hours with the food removed and new bedding in the cages. The food withdrawn for the entire experimental duration. The body weights and basal blood glucose concentrations (using Glu cometer Contour next EZ, Ascensia Diabetes Care US, Inc.) were measured and divided into 2 groups (n=4/group). Following the basal measurements, the mice received ip injections 0.75 U/Kg body weight of either human insulin (Novolin ReliOn) or SCS-Ins dissolved in saline. The blood glucose concentrations were measured at 10, 20, 30 and 60 min after injection.
Supplementary Material
ACKNOWLEDGMENT
We thank Prof. Dr. Michael S. Kay, Dr. Xiaochun Xiong and Dr. Landa Purushottam for helpful discussions. We thank NIDDK for funding support (R01DK120430). Dr. Nan Zheng is an American Diabetes Association (ADA) postdoctoral fellow (#1-19-PDF- 110).
ABBREVIATIONS USED
- Acm
acetamidomethyl
- ACN
acetonitrile
- 2-CTC
2-chlorotrityl chloride
- DCM
dichloromethane
- DIEA
diisopropylethylamine
- DTDP
2,2’-Dithiodipyridine
- FA
formic acid
- HATU
O-Benzotriazole-N,N,N’,N’-tetramethyluronium hexafluorophosphate
- Ins
insulin
- ip
intraperitoneal
- Mob
4-methoxybenzyl
- NMP
methylpyrrolidone
- RP
reversed phase
- SD
standard deviation
- SPPS
solid phase peptide synthesis
- TCEP.HCl
Tris(2-carboxy-ethyl)phosphine hydrochloride
- TFA
trifluoroacetic acid
- TIPS
triisopropylsilane
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI:
HPLC trace, LCMS characterization of intermediates and Glu-C digestion experiment. (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Ryle AP; Sanger F; Smith LF; Kitai R The Disulphide Bonds of Insulin. Biochem. J 1955, 60, 541–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Banting FG; Best CH The Internal Secretion of the Pancreas. J. Lab. Clin. Med 1922, 7, 251–266. [PubMed] [Google Scholar]
- (3).(a) Tibaldi JM Evolution of Insulin: From Human to Analog. Am J Med 2014, 127, 25–38. [DOI] [PubMed] [Google Scholar]; (b) Zaykov AN; Mayer JP; Di- Marchi RD Pursuit of a Perfect Insulin. Nat Rev Drug Discov. 2016, 15, 425–439. [DOI] [PubMed] [Google Scholar]
- (4).(a) Sluzky V; Tamada JA; Klibanov AM; Langer R Kinetics of Insulin Aggregation in Aqueous Solutions upon Agitation in the Presence of Hydrophobic Surfaces. Proc. Natl. Acad. Sci. U. S. A 1991, 88, 9377–9381. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Brange J; Langkjaer L; Havelund S; Volund A Chemical stability of insulin. 1. Hydrolytic Degradation during Storage of Pharmaceutical Preparations. Pharm Res. 1992, 9, 715–726. [DOI] [PubMed] [Google Scholar]; (c) Brange J; Havelund S; Hougaard P Chemical Stability of Insulin. 2. Formation of Higher Molecular Weight Transformation Products during Storage of Pharmaceutical Preparations. Pharm Res. 1992, 9, 727–734. [DOI] [PubMed] [Google Scholar]
- (5).Weiss M Design of Ultra-stable Insulin Analogues for the Developing World. J. Health Spec 2013, 1, 59–70. [Google Scholar]
- (6).(a) Vinther TN; Norrman M; Ribel U; Huus K; Schlein M; Steensgaard DB; Pedersen TA; Pettersson I; Ludvigsen S; Kjeldsen T; Jensen KJ; Hubalek F Insulin Analog with Additional Disulfide Bond has Increased Stability and Preserved Activity. Protein Sci 2013, 22, 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Brunel FM; Mayer JP; Gelfanov VM; Zaykov AN; Finan B; Perez-Tilve D; DiMarchi RD A Disulfide Scan of Insulin by [3+ 1] Methodology exhibits Site-specific Influence on Bioactivity. ACS Chem Biol. 2019, 14, 1829–1835. [DOI] [PubMed] [Google Scholar]
- (7).(a) Hua QX; Nakagawa SH; Jia W; Huang K; Phillips NB; Hu SQ; Weiss MA Design of an Active Ultrastable Single-chain Insulin Analog: Synthesis, Structure, and Therapeutic Implications. J Biol Chem. 2008, 283, 14703–14716. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Glidden MD; Aldabbagh K; Phillips NB; Carr K; Chen YS; Whittaker J; Phillips M; Wickramasinghe NP; Rege N; Swain M; Peng Y; Yang Y; Lawrence MC; Yee VC; Ismail-Beigi F; Weiss MA An Ultra-stable Single-chain Insulin Analog Resists Thermal Inactivation and Exhibits Biological Signaling Duration Equivalent to the Native Protein. J Biol Chem. 2018, 293, 47–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Guan X; Chaffey PK; Wei X; Gulbranson DR; Ruan Y; Wang X; Li Y; Ouyang Y; Chen L; Zeng C; Koelsch TN; Tran AH; Liang W; Shen J; Tan Z Chemically Precise Glycoengineering Improves Human Insulin. ACS Chem Biol. 2018, 13, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Lieblich SA; Fang KY; Cahn JKB; Rawson J; LeBon J; Ku HT; Tirrell DA 4S-Hydroxylation of Insulin at ProB28 Accelerates Hexamer Dissociation and Delays Fibrillation. J Am Chem Soc. 2017, 139, 8384–8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) Gori A; Gagni P; Rinaldi S Disulfide Bond Mimetics: Strategies and Challenges. Chem. Eur. J 2017, 23, 14987–14995. [DOI] [PubMed] [Google Scholar]; (b) Karas J; Shabanpoor F; Hossain MA; Gardiner J; Separovic F; Wade JD; Scanlon DB Total Chemical Synthesis of a Heterodimeric Interchain Bis-lactam-linked Peptide: Application to an Analogue of Human Insulin-like Peptide 3. Int. J. Pept 2013, 2013, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Karas JA; Patil NA; Tailhades J; Sani MA; Scanlon DB; Forbes BE; Gardiner J; Separovic F; Wade JD; Hossain MA Total Chemical Synthesis of an Intra-A-chain Cystathionine Human Insulin Analogue with Enhanced Thermal Stability. Angew. Chem., Int. Ed 2016, 55, 14743–14747. [DOI] [PubMed] [Google Scholar]
- (12).Ong SC; Belgi A; van Lierop B; Delaine C; Andrikopoulos S; MacRaild CA; Norton RS; Haworth NL; Robinson AJ; Forbes BE Probing the Correlation between Insulin Activity and Structural Stability through Introduction of the Rigid A6–A11 Bond. J Biol Chem 2018, 293, 11928–11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Weil-Ktorza O; Rege N; Lansky S; Shalev DE; Shoham G; Weiss MA; Metanis N Substitution of an Internal Disulfide Bridge with a Diselenide Enhances both Foldability and Stability of Human Insulin. Chem. Eur. J 2019, 25, 8513–8521. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Safavi- Hemami H; Gajewiak J; Karanth S; Robinson SD; Ueberheide B; Douglass AD; Schlegel A; Imperial JS; Wat-kins M; Bandyopadhyay PK; Yandell M; Li Q; Purcell AW; Norton RS; Ellgaard L; Olivera BM Specialized Insulin is used for Chemical Warfare by Fish-hunting Cone Snails. Proc Natl Acad Sci U S A. 2015, 112, 1743–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Arai K; Takei T; Okumura M; Watanabe S; Amagai Y; Asahina Y; Moroder L; Hojo H; Inaba K; Iwaoka M Preparation of Selenoinsulin as a Long-lasting Insulin Analogue. Angew. Chem., Int. Ed 2017, 56, 5522–5526. [DOI] [PubMed] [Google Scholar]
- (15).Williams GM; Lee K; Li X; Cooper GJ; Brimble MA Replacement of the CysA7–CysB7 Disulfide Bond with a 1, 2, 3-Triazole Linker Causes Unfolding in Insulin Glargine. Org Biomol Chem. 2015, 13, 4059–4063. [DOI] [PubMed] [Google Scholar]
- (16).(a) Wang Y; Bruno BJ; Cornillie S; Nogieira JM; Chen D; Cheatham, 3rd, T. E.; Lim, C. S.; Chou, D. H. Application of Thiolyne/thiol-ene Reactions for Peptide and Protein Macrocyclizations. Chem. Eur. J 2017, 23, 7087–7092. [DOI] [PubMed] [Google Scholar]; (b) Wang Y; Chou DH A thiolene Coupling Approach to Native Peptide Stapling and Macrocyclization. Angew. Chem., Int. Ed 2015, 54, 10931–10934. [DOI] [PubMed] [Google Scholar]
- (17).Kourra C; Cramer N Converting Disulfide Bridges in Native Peptides to Stable Methylene Thioacetals. Chem Sci. 2016, 7, 7007–7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).(a) Hossain MA; Wade JD Novel Methods for the Chemical Synthesis of Insulin Superfamily Peptides and of Analogues Containing Disulfide Isosteres. Acc Chem Res. 2017, 50, 2116–2127. [DOI] [PubMed] [Google Scholar]; (b) Liu F; Li P; Gelfanov V; Mayer J; DiMarchi RD Synthetic Advances in Insulin-like Peptides Enable Novel Bioactivity. Acc Chem Res. 2017, 50, 1855–1865. [DOI] [PubMed] [Google Scholar]
- (19).(a) Hossain MA; Belgi A; Lin F; Zhang S; Shabanpoor F; Chan L; Belyea C; Truong HT; Blair AR; Andrikopoulos S; Tregear GW; Wade JD Use of a Temporary “Solubilizing” Peptide Tag for the Fmoc Solid-phase Synthesis of Human Insulin Glargine via Use of Regioselective Disulfide Bond Formation. Bioconjug Chem. 2009, 20, 1390–1396. [DOI] [PubMed] [Google Scholar]; (b) Williams GM; Cooper GJ; Lee K; Whiting L; Brimble MA Synthesis of the IGF-II-like Hormone Vesiculin Using Regioselective Formation of Disulfide Bonds. Org Biomol Chem. 2013, 11, 3145–3150. [DOI] [PubMed] [Google Scholar]; (c) Yoshiya T; Taniguchi A; Sohma Y; Fukao F; Nakamura S; Abe N; Ito N; Skwarczynski M; Kimura T; Hayashi Y; Kiso Y “O-Acyl Isopeptide Method” for Peptide Synthesis: Synthesis of Forty Kinds of “O-acyl Isodipeptide Unit” Boc-Ser/Thr (Fmoc-Xaa)-OH. Org Biomol Chem. 2007, 5, 1720–1730. [DOI] [PubMed] [Google Scholar]; (d) Boross GN; Shimura S; Besenius M; Tennagels N; Rossen K; Wagner M; Bode JW Facile Folding of Insulin Variants Bearing a Prosthetic C-peptide Prepared by α-ketoacid-hydroxylamine (KAHA) Ligation. Chem Sci. 2018, 9, 8388–8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Liu F; Luo EY; Flora DB; Mezo AR A Synthetic Route to Human Insulin Using Isoacyl Peptides. Angew. Chem., Int. Ed 2014, 53, 3983–3987. [DOI] [PubMed] [Google Scholar]
- (21).(a) Jacobsen MT; Petersen ME; Ye X; Galibert M; Lorimer GH; Aucagne V; Kay MS A Helping Hand to Overcome Solubility Challenges in Chemical Protein Synthesis. J Am Chem Soc. 2016, 138, 11775–11782. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Disotuar MM; Petersen ME; Nogueira JM; Kay MS; Chou DH Synthesis of Hydrophobic Insulin-based Peptides Using a Helping Hand Strategy. Org Biomol Chem. 2019, 17, 1703–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Fulcher JM; Petersen ME; Giesler RJ; Cruz ZS; Eckert DM; Francis JN; Kawamoto EM; Jacobsen MT; Kay MS Chemical Synthesis of Shiga Toxin Subunit B Using a Next-generation Traceless “Helping Hand” Solubilizing Tag. ChemRxiv. 2019, DOI: 10.26434/chemrxiv.9759314.v1 [DOI] [PubMed] [Google Scholar]
- (23).Gongora-Benitez M; Mendive-Tapia L; Ramos-Tomillero I; Breman AC; Tulla-Puche J; Albericio F Acid-Labile Cys-Protecting Groups for the Fmoc/tBu Strategy: Filling the Gap. Org Lett. 2012, 14, 5472–5475. [DOI] [PubMed] [Google Scholar]
- (24).Liu F; Luo EY; Flora DB; Mayer JP Concise Synthetic Routes to Human Insulin. Org Lett. 2013, 15, 960–963. [DOI] [PubMed] [Google Scholar]
- (25).Hua QX; Chu YC; Jia W; Phillips NF; Wang RY; Katsoyannis PG; Weiss MA Mechanism of Insulin Chain Combination Asymmetric Roles of A-chain α-helices in Disulfide Pairing. J Biol Chem. 2002, 277, 43443–43453. [DOI] [PubMed] [Google Scholar]
- (26).(a) Qiao ZS; Guo ZY; Feng YM Putative Disulfide- Forming Pathway of Porcine Insulin Precursor During Its Refolding In Vitro. Biochemistry. 2001, 40, 2662–2668. [DOI] [PubMed] [Google Scholar]; (b) Arai K; Takei T; Shinozaki R; Noguchi M; Fujisawa S; Katayama H; Moroder L; Ando S; Okumura M; Inaba K; Hojo H; Iwaoka M Characterization and Optimization of Two-chain Folding Pathways of Insulin via Native Chain Assembly. Communications Chemistry. 2018, 1, 26. [Google Scholar]
- (27).Webber MJ; Appel EA; Vinciguerra B; Cortinas AB; Thapa LS; Jhunjhunwala S; Isaacs L; Langer R; Anderson DG Supramolecular PEGylation of Biopharmaceuticals. Proc Natl Acad Sci U S A. 2016, 113, 14189–14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Hua QX; Weiss MA Mechanism of Insulin Fibrillation the Structure of Insulin under Amyloidogenic Conditions Resembles a Protein-folding Intermediate. J Biol Chem. 2004, 279, 21449–21460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


