Fig. 2.
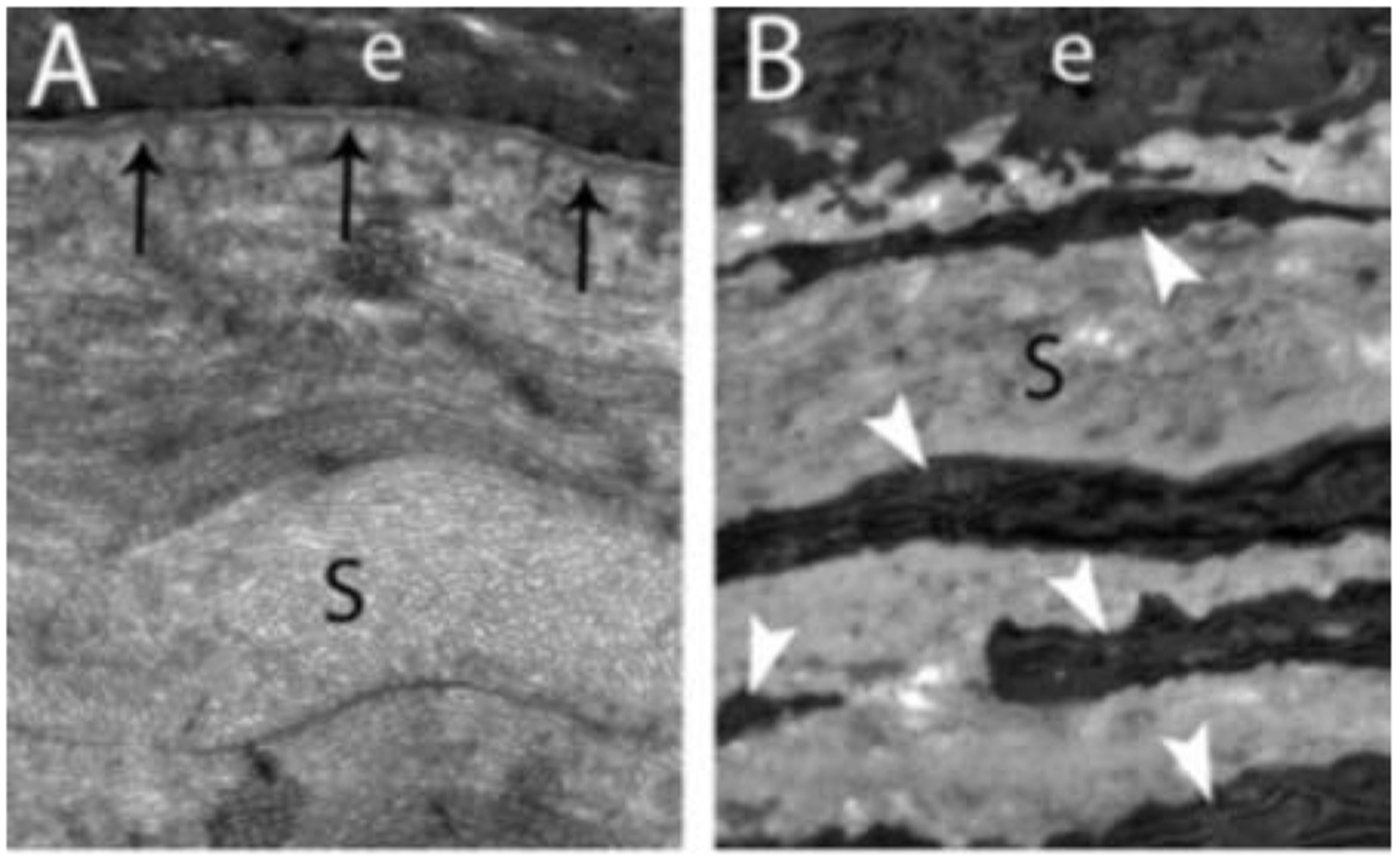
Schematic model of normal unwounded corneal epithelial basement membrane (EBM) and injured EBM with defective regeneration associated with anterior stromal myofibroblast development and fibrosis. Normal unwounded cornea (A) with intact epithelial basement membrane (EBM) comprised of laminins 511, 521, 111, 332, etc., in addition to perlecan, nidogen-1, nidogen-2, and other components not depicted, such as collagen type IV. The underlying stroma is populated with keratocytes within the highly-organized, transparent stromal collagen lamellae. Epithelial transforming growth factor beta (TGFβ and platelet-derived growth factor (PDGF) are blocked from penetration into the underlying stroma by binding components such as perlecan, nidogen and collagen type IV within the normal EBM. B) After severe epithelial-stromal injuries, such as infections, trauma or high-correction photorefractive keratectomy, the epithelium and EBM are disrupted and TGFβ and PDGF are activated and penetrate the underlying stroma at sufficient concentrations to drive the development of myofibroblasts from keratocyte-derived and bone marrow-derived (fibrocyte) precursors. Myofibroblasts are themselves opaque relative to keratocytes and secrete disordered collagen type 1, collagen type 3 and other matrix materials that disrupt the normal stromal lamellae to produce corneal opacity or scarring. C) Over months to years following the initial injury, keratocytes penetrate the anterior stromal myofibroblasts and facilitate EBM regeneration via the production of perlecan and nidogens in coordination with overlying epithelial cells producing other EBM components, such as laminins and collagen type IV. We hypothesize that once the nascent laminin layer containing laminins 111, 511, 521 and 332 is produced by the epithelium, more posterior EBM components must, at least in part, be derived from keratocytes and/or corneal fibroblasts to fully-regenerate the normal EBM. The resulting decrease in TGFβ and PDGF penetration from the epithelium into the stroma triggers myofibroblast apoptosis via unopposed paracrine IL-1α from adjacent keratocytes and/or autocrine IL-1α produced by the myofibroblasts themselves (autocrine suicide). This process begins in a random spotty distribution within the stromal opacity to produce clear areas of stroma called “lacunae” (Fig. 5A and 5B) that enlarge and coalesce over weeks to months to fully restore transparency. D) In some corneas, depending on the severity of the injury, myofibroblasts undergo apoptosis and keratocytes repopulate the anterior stroma and reabsorb disorganized collagens and other matrix materials secreted by the myofibroblasts, thereby restoring the normal morphology of the collagen lamellae and transparency. Revised with permission from Wilson et al., Matrix Biol 64, 17–26.
