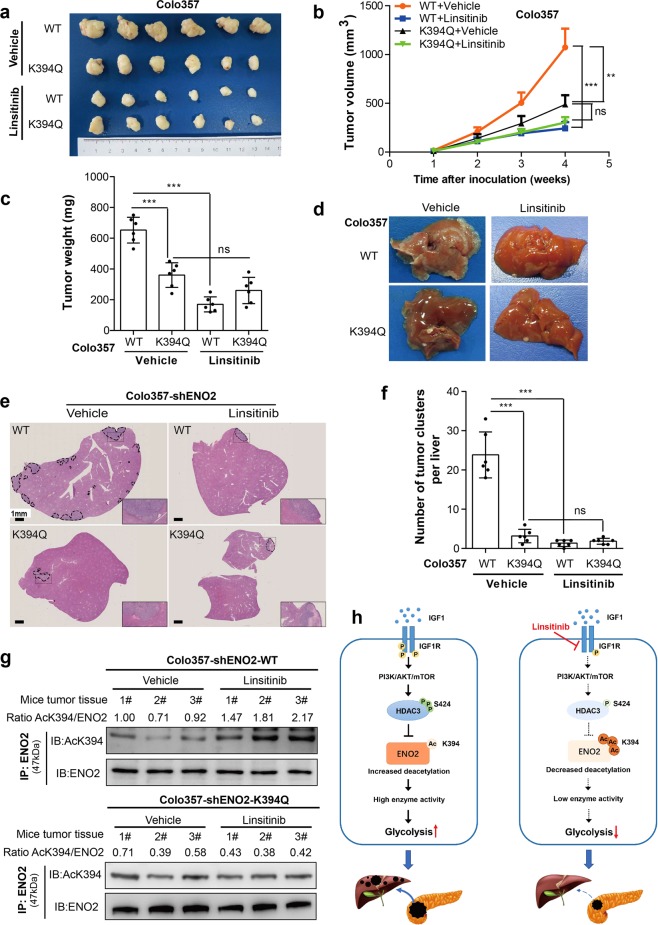
Fig. 6.
Inhibition of IGF-1R protects PDAC cells from IGF-1-induced tumor growth and metastasis. a–c In subcutaneous xenograft mouse models, Colo357 cells with ENO2 knockdown stably expressing wild-type ENO2 or K394Q mutants were subcutaneously injected into nude mice (n = 6 per group). When the tumor reached 100 mm3, the mice were treated with either 50 mg/kg linsitinib or vehicle once daily on a 5 days on and 3 days off cycle by oral gavage. Tumor volume (a), tumor growth curves (b) and tumor weight (c) were analyzed. d–f In metastatic mouse models, Colo357 cells (2.5 × 105 cells per mouse, n = 6 per group) as indicated were intrasplenically injected, and mice were subsequently treated with either 50 mg/kg linsitinib or vehicle once daily on a 5 days on and 3 days off cycle by oral gavage. Metastatic nodule detection (d), H&E staining (e) and analysis of metastatic lesions (f) in the livers were performed (scale bar = 1 mm). g Xenograft tumor tissues as indicated were lysed and subjected to IP and western blot with anti-ENO2 and anti-AcK394 antibodies. K394 acetylation levels were normalized against ENO2 protein levels. h A working model showing that ENO2 K394 deacetylation plays a key role in promoting the metastasis of PDAC. Enolase 2 (ENO2), a key glycolytic enzyme, is acetylated by PCAF and deacetylated by HDAC3 at K394. The IGF-1/PI3K/Akt/mTOR pathway induces K394 deacetylation and stimulates ENO2 activity by increasing the phosphorylation of HDAC3 at S424, thereby promoting the growth and metastasis of PDAC. Linsitinib, an oral small-molecule inhibitor of IGF-1R, could inhibit IGF-1-induced ENO2 deacetylation and provide a promising strategy to prevent the development and progression of PDAC. Error bars represent the mean ± SD, and the dots represent the value of each experiment; **P < 0.01, ***P < 0.001, ns: no significance. Two-way ANOVA followed by Bonferroni’s post hoc test was employed in (b), and one-way ANOVA followed by Bonferroni’s post hoc test was employed in (c) and (f)