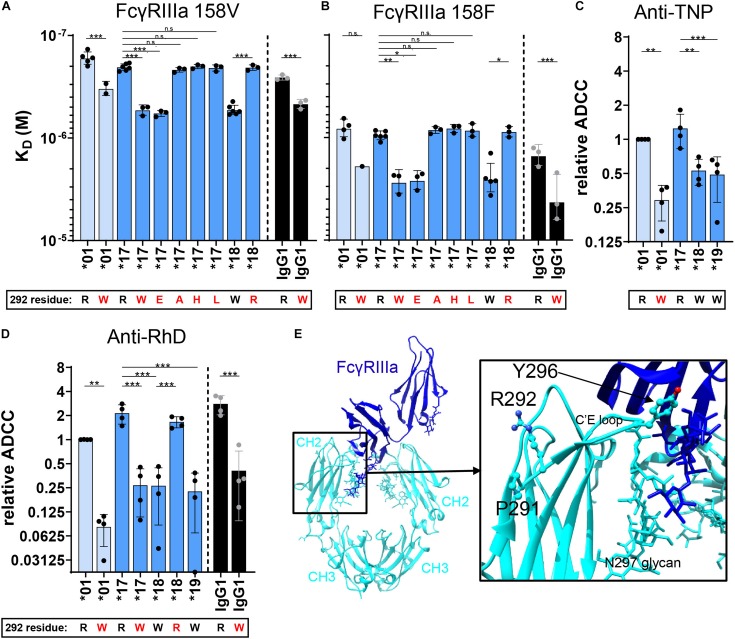
FIGURE 4.
FcγRIIIa binding and ADCC capacity of 292-mutated IgG allotypes. The affinity (KD) of 292–mutant anti-RhD IgG allotypes for (A) FcγRIIIa 158V and (B) FcγRIIIa 158F was determined by SPR. Error bars indicate SEM of ≥2 independent measurements. ADCC activity was assessed for 292-mutant (C) anti-TNP allotypes and (D) anti-RhD antibodies using NK cells from four different donors. The relative ADCC capacity compared to IgG3 allotype *01 is plotted (original ADCC data in Supplementary Figure S4). IgG3 allotypes and 292-mutants with a long hinge are displayed in light blue, an intermediate hinge in blue, a short hinge in dark blue and IgG1 in black. In all graphs the amino acid at residue 292 (single-letter code) for each antibody is indicated at the x-axis below the IgG3 allotype number, where 292-mutated antibodies are displayed with a red letter. Statistical comparison between groups was performed using a One-way ANOVA with Sidak’s multiple comparisons test, and significant differences are indicated with asterisks: *p < 0.05, **p < 0.01, ***p < 0.001. (E) Crystal structure of fucosylated IgG1 Fc (cyan) in complex with FcγRIIIa (blue) (PDB; 3SGJ) (53). Residues Pro-291, Arg-292 and Tyr-296 are displayed in a ball-and-stick model.