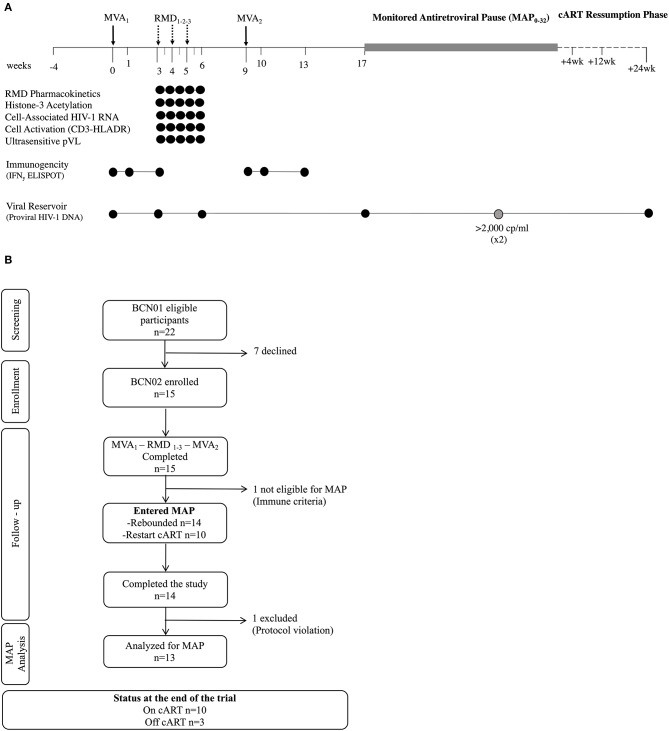
Figure 1.
Trial design. (A) Schematic study design. (B) Consolidated Standards of Reporting Trials (CONSORT) flow diagram for the trial. *Viral rebound during MAP was defined as pVL >20 copies/ml and #criteria for ART resumption included pVL over 2,000 copies/ml in two consecutive determinations, CD4+ cell counts decrease over 50% and/or below 500 cells/mm3 and/or development of clinical symptoms suggestive of an acute retroviral syndrome. MVA, MVA.HIVconsv vaccine; RMD, romidepsin; MAP, monitored antiretroviral pause; ART, antiretroviral therapy; pVL, plasma HIV-1 viral load.