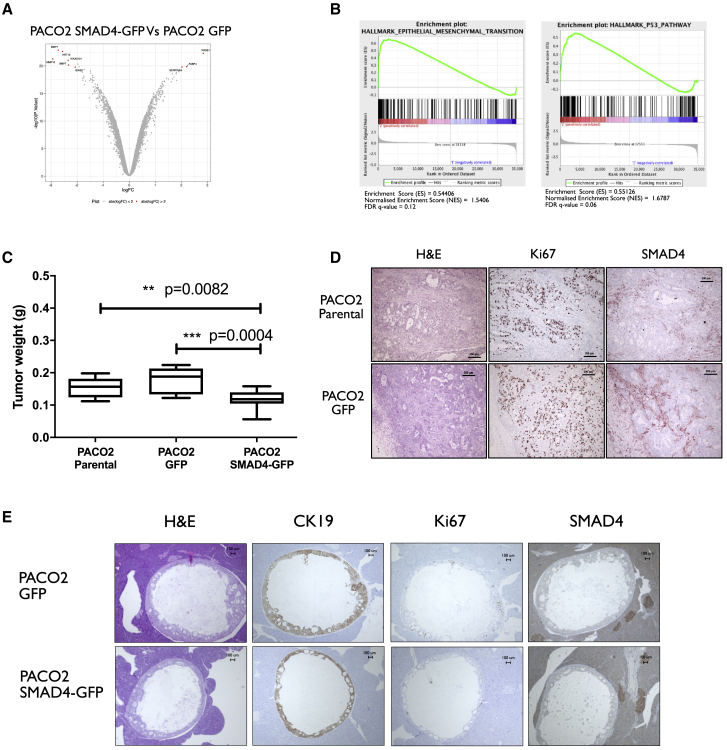
Figure 5.
In Vitro Restoration of SMAD4 Functionality in Primary Models
(A and B) Differentially expressed genes were analyzed with a >2-fold and less than −2-fold log fold change (FC) (p < 0.05) (A) and further validated in gene set enrichment analysis (GSEA) (B), where PACO2 SMAD4 cells showed strong enrichment for the hallmarks underlying the epithelial-to-mesenchymal transition and the activation of the TGF-β pathways when compared to the PACO2 GFP control line. (C) The impact of SMAD4 on tumor growth was evaluated in vivo by injecting 5 × 105 PACO2 parental, PACO2 GPF, and PACO2 SMAD4 cells orthotopically into the pancreas of NSG mice. The weight of the pancreas was used as a measure of tumor growth, and we showed that mice injected with PACO2 cells had significantly lighter organs than did animals injected with the control cell lines (n = 10 for PACO2 parental, n = 12 for PACO2 GFP and SMAD4 analyzed by one-way ANOVA followed by the Tukey’s post hoc test for multiple comparisons). (D) Primary tumors obtained from the orthotopic injection of not modified and PACO2 GFP cells were assessed for morphology with H&E staining, proliferation via staining with the proliferative marker Ki67, and for the expression of SMAD4. The tumors formed from PACO2 modified with the reporter gene GFP showed a defined ductal differentiation typical of pancreatic cancers with a high proliferate rate and negative expression for SMAD4 identical to unmodified parental PACO2 cells. (E) Cells where SMAD4 functionality was restored did not form tumors when engrafted into mice. The outgrowing masses originated from human cells (CK19 positive), were actively proliferating (Ki67 positive), and did not stain positive for SMAD4 expression.