Fig. 1.
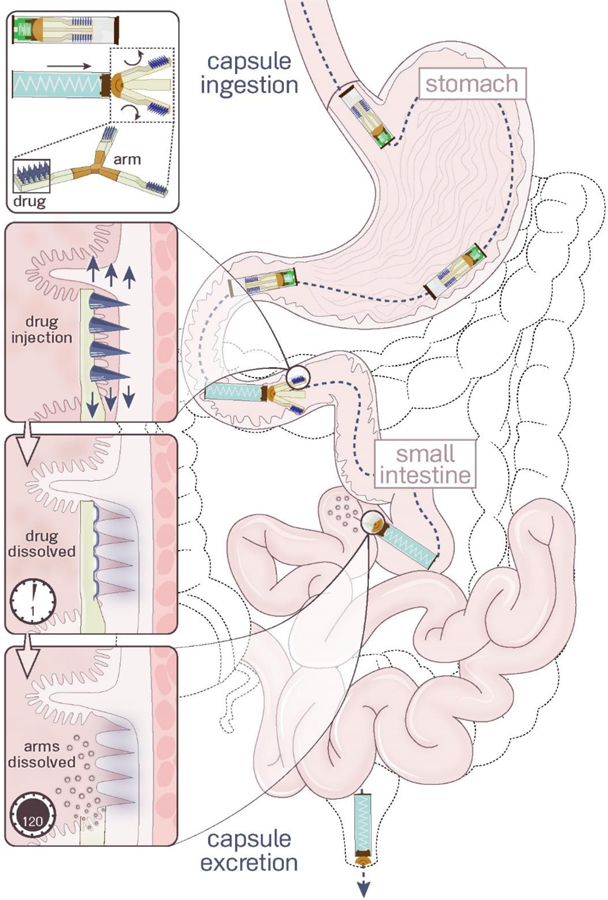
Luminal Unfolding Microneedle Injector (LUMI) design. LUMI devices were ingested in waterproof enteric capsules. They actuated and unfolded in the small intestine, injecting drug loaded microneedles into the tissue wall. The microneedle patches and arms dissolved within several hours. The non-degradable parts of the device passed through the GI tract and were excreted.
