Fig. 2.
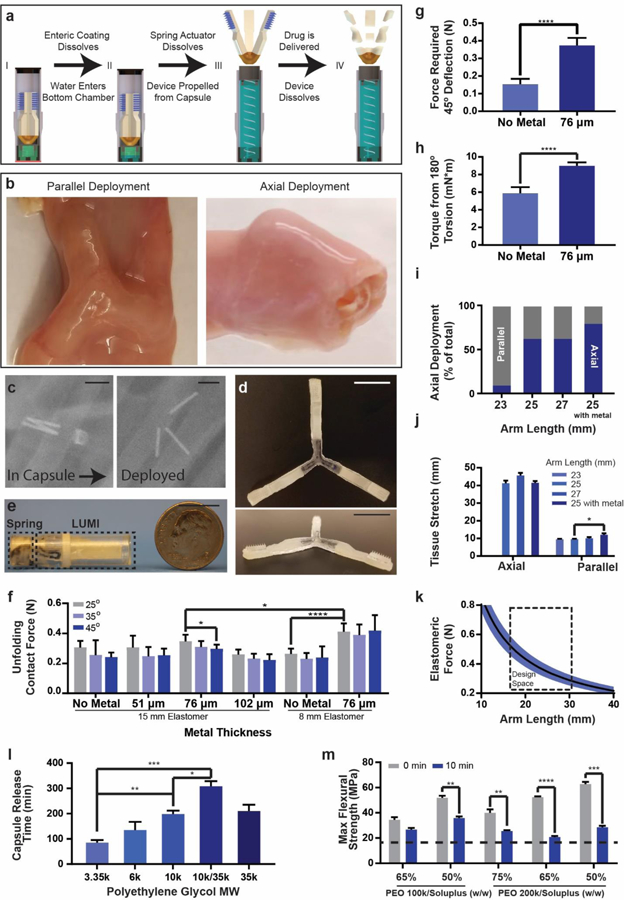
LUMI fabrication and design specifications. (a) The LUMI device was housed inside of a waterproof chamber until it reached the small intestine. After delivering the LUMI, the capsule broke apart into small pieces and passed through the GI tract. (b) LUMI devices opened up in multiple orientations in the small intestine, including in the parallel or axial directions shown in this figure. (c) X-rays confirmed that the capsule actuated and released the LUMI device within 2 hours. The metal rods were used for imaging purposes and were not part of the final design. (d) Photo of an unfolded and (e) encapsulated LUMI. (f) Unfolding contact force applied by the arm (n=9). (g) Forces required for arm deflection and (h) torsion (n=9). (i) Percent of devices deployed axially in vivo (n=15). (j) Tissue stretch from unfolding (n=9). (k) LUMI design space based on arm length and elastomeric force beneficial for administration. (l) Capsule Release time is dependent on molecular weight of PEG coating (n=3). (m) Arm flexural strength before and after dissolution in simulated intestinal fluid at 37°C. The dotted line represented the calculated flexural stress required to break the LUMI arm (n=3). (Error Bars=SD; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001) Scale Bars are 1 cm.
